
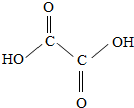
 ����С��Ϻ��һ����ʳ������Щ�̷��á�ϴϺ�ۡ�����ϴ��Ϻ������࣬��ϴϺ�ۡ��к��в��ᣬ������Ϊ�����10000�����������Σ��������ͼΪ����Ľṹʽ���Իش��������⣺
����С��Ϻ��һ����ʳ������Щ�̷��á�ϴϺ�ۡ�����ϴ��Ϻ������࣬��ϴϺ�ۡ��к��в��ᣬ������Ϊ�����10000�����������Σ��������ͼΪ����Ľṹʽ���Իش��������⣺


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A�������Ͷ�����̼-ȼ�ŵ�ľ�� |
| B���������ƹ��������粒���-ˮ |
| C��Ӳˮ������ˮ-����ˮ |
| D������������Һ��ʯ��ˮ-��̪��Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ���� | ���� | |
| ������̼ CO2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ʵ�鲽�� | ʵ������ | ʵ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
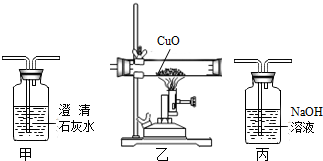
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��ͼ��ÿ��ֱ�����˵�������һ�������¶����Է�����ѧ��Ӧ�����мס��ҡ��������ֱ�ΪFe��BaCl2��Na2CO3���������������е�һ�֣�
��ͼ��ÿ��ֱ�����˵�������һ�������¶����Է�����ѧ��Ӧ�����мס��ҡ��������ֱ�ΪFe��BaCl2��Na2CO3���������������е�һ�֣��鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com