


 �ظ���ʦ�㲦ϵ�д�
�ظ���ʦ�㲦ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
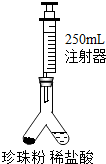 ��2013?������һģ��������������õ����յȹ�Ч�����������ϲ����������ʾ�������к�̼���80-93%��������4-14%��ˮ��2-4%��ʮ���ְ����ᡢ28����Ԫ�أ�Ȼ��һЩ���������û��յı��Ǽ��Ϲ�ҵ�������������Ƶ�ҩˮ������ϴ����ɹ�Ժ�ֱ�Ӽӹ����ۣ�����Ҫ�ɷ��ǣ�̼��ƣ������������������ƣ�������ij��ѧ��ȤС����Ʊ��������飬���ⶨ������̼��ƺ�����ʵ��̽��������
��2013?������һģ��������������õ����յȹ�Ч�����������ϲ����������ʾ�������к�̼���80-93%��������4-14%��ˮ��2-4%��ʮ���ְ����ᡢ28����Ԫ�أ�Ȼ��һЩ���������û��յı��Ǽ��Ϲ�ҵ�������������Ƶ�ҩˮ������ϴ����ɹ�Ժ�ֱ�Ӽӹ����ۣ�����Ҫ�ɷ��ǣ�̼��ƣ������������������ƣ�������ij��ѧ��ȤС����Ʊ��������飬���ⶨ������̼��ƺ�����ʵ��̽���������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com