��������Ҫ�Ľ������ϣ���ش������й����⣺
��1����������Ʒ����;�У����ý��������Ե���
B
B
������ĸ����ͬ����
A��

���� B��

���� C��

ͭ˿ D��

ˮ��ͷ
��2�������й�������������ȷ����
C
C
��
A������������ɫ�����ʵؽ��� B�������������еġ���������Ϊ���Ͻ�
C������������ȼ�����ɺ�ɫ�������� D���ؿ��е������Ի�������ʽ����
��3����������������ʴ������������ÿ������ʴ�����ϵĽ������ϣ��൱���������20%��40%��Ϊ��ֹ������Ʒ����ʴ�����÷�����
ˢ���������Ϳ�ͣ��Ƹ����ƳɺϽ��
ˢ���������Ϳ�ͣ��Ƹ����ƳɺϽ��
��
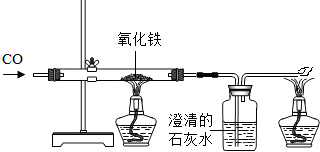
��4������ÿ�����Ȼ������ȡ�����Ľ���������������¯�з����ķ�Ӧ�У�
��Fe
2O
3+3CO
2Fe+3CO
2����C+O
2CO
2����CO
2+C
2CO��
��CaCO
3CaO+CO
2���� ��CaO+SiO
2CaSiO
3��
�������Ϸ�Ӧ�ƶϣ�������ԭ����Ҫ��
����ʯ����̿��ʯ��ʯ�������������Ⱥ�
����ʯ����̿��ʯ��ʯ�������������Ⱥ�
����ԭ����ʯ�ķ�Ӧ��
��
��
�������ķ�Ӧ��
�ܺ͢�
�ܺ͢�
��������Դ�ķ�Ӧ��
��
��
��������ţ�

 ���� B��
���� B�� ���� C��
���� C�� ͭ˿ D��
ͭ˿ D�� ˮ��ͷ
ˮ��ͷ

 ��ǰ�κ�ͬ����ϰϵ�д�
��ǰ�κ�ͬ����ϰϵ�д� ����С��ҵϵ�д�
����С��ҵϵ�д� �Ƹ�С״Ԫ����������ϰ��ϵ�д�
�Ƹ�С״Ԫ����������ϰ��ϵ�д� �ɹ�ѵ���ƻ�ϵ�д�
�ɹ�ѵ���ƻ�ϵ�д� ����ѵ����ֱͨ�п�����ϵ�д�
����ѵ����ֱͨ�п�����ϵ�д� һ���㶨ϵ�д�
һ���㶨ϵ�д� ��У��ҵ��ϵ�д�
��У��ҵ��ϵ�д�

 ��������Ҫ�Ľ������ϣ��ڽ��컴����ˮ�ɻ���ʱ�������˴����ĸ�����
��������Ҫ�Ľ������ϣ��ڽ��컴����ˮ�ɻ���ʱ�������˴����ĸ�����