

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
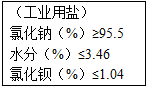
| ����ҵ���Σ� �Ȼ��ƣ�%����95.5 ˮ�֣�%����3.46 �Ȼ�����%����1.04��1��100g�ù�ҵ���к��Ȼ����������� ��2��ͨ�����㣬�жϴ˹�ҵ�����Ȼ��Ƶ����������Ƿ���ϲ�Ʒ����ָ�ꣿ �鿴�𰸺ͽ���>> ��Ŀ�����л�ѧ ��Դ��2010��������Ͽ����п���ѧһģ�Ծ��������棩 ���ͣ������ ��ͼ����ij��ҵ�β�Ʒ����ָ�꣮Ϊ�˲ⶨ�ù�ҵ�����Ȼ��Ƶ�����������ȡ100g�ù�ҵ�ν���ʵ�飺�ٲ��ˮ����������Ϊ3.36%������̼�������ⶨ�����Ȼ���������ʱ���õ�0.985g��������̼�������Ȼ�����Ӧ�Ļ�ѧ����ʽ�� BaCl2+Na2CO3=BaCO3��+2NaCl��
��2��ͨ�����㣬�жϴ˹�ҵ�����Ȼ��Ƶ����������Ƿ���ϲ�Ʒ����ָ�ꣿ �鿴�𰸺ͽ���>> ��Ŀ�����л�ѧ ��Դ��2012���п���ѧģ���Ծ���2���������棩 ���ͣ������ ��ͼ����ij��ҵ�β�Ʒ����ָ�꣮Ϊ�˲ⶨ�ù�ҵ�����Ȼ��Ƶ�����������ȡ100g�ù�ҵ�ν���ʵ�飺�ٲ��ˮ����������Ϊ3.36%������̼�������ⶨ�����Ȼ���������ʱ���õ�0.985g��������̼�������Ȼ�����Ӧ�Ļ�ѧ����ʽ�� BaCl2+Na2CO3=BaCO3��+2NaCl��
��2��ͨ�����㣬�жϴ˹�ҵ�����Ȼ��Ƶ����������Ƿ���ϲ�Ʒ����ָ�ꣿ �鿴�𰸺ͽ���>> ��Ŀ�����л�ѧ ��Դ��2007������������п���ѧģ���Ծ���һ���������棩 ���ͣ������ ��2004?���ݣ���ͼ����ij��ҵ�β�Ʒ����ָ�꣮Ϊ�˲ⶨ�ù�ҵ�����Ȼ��Ƶ�����������ȡ100g�ù�ҵ�ν���ʵ�飺�ٲ��ˮ����������Ϊ3.36%������̼�������ⶨ�����Ȼ���������ʱ���õ�0.985g��������̼�������Ȼ�����Ӧ�Ļ�ѧ����ʽ�� BaCl2+Na2CO3=BaCO3��+2NaCl��
��2��ͨ�����㣬�жϴ˹�ҵ�����Ȼ��Ƶ����������Ƿ���ϲ�Ʒ����ָ�ꣿ �鿴�𰸺ͽ���>> ��Ŀ�����л�ѧ ��Դ��2010��㶫ʡ�麣�ж���ʵ����ѧ�п���ѧ��ģ�Ծ��������棩 ���ͣ������ 26����2004?���ݣ���ͼ����ij��ҵ�β�Ʒ����ָ�꣮Ϊ�˲ⶨ�ù�ҵ�����Ȼ��Ƶ�����������ȡ100g�ù�ҵ�ν���ʵ�飺�ٲ��ˮ����������Ϊ3.36%������̼�������ⶨ�����Ȼ���������ʱ���õ�0.985g��������̼�������Ȼ�����Ӧ�Ļ�ѧ����ʽ�� BaCl2+Na2CO3=BaCO3��+2NaCl��
��2��ͨ�����㣬�жϴ˹�ҵ�����Ȼ��Ƶ����������Ƿ���ϲ�Ʒ����ָ�ꣿ �鿴�𰸺ͽ���>> ͬ����ϰ��� ����ѧУ��ѡ - ��ϰ���б� - �����б� ����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר�� Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com��Ȩ��������վ�������£�ͼƬ��Դ�����磬����Ȩ����Ȩ��ԭ�������У�ת�������ַ���Ȩ��������Ȩ����������������֪�����ǽ����촦������ϵqq��3310059649�� ICP�������: ��ICP��07509807��-10 ����������42018502000812�� |