
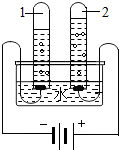
 ˮ����Һ�������������������������ʮ����Ҫ�����ã�
ˮ����Һ�������������������������ʮ����Ҫ�����ã�| �¶�/�� | 0 | 20 | 40 | 60 | 80 | 100 | |
| �ܽ�� /g | NaCl | 35.7 | 36.0 | 36.6 | 37.3 | 38.4 | 39.8 |
| NH4Cl | 29.4 | 37.2 | 45.8 | 55.2 | 65.6 | 77.3 | |
| ||


 ���Ž�������С״Ԫϵ�д�
���Ž�������С״Ԫϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
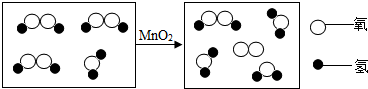
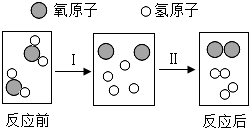 ����������Ƽ��������ȶ��벻����ѧ��
����������Ƽ��������ȶ��벻����ѧ�� ����ʾһ�������ӣ���
����ʾһ�������ӣ��� ����ʾ
����ʾ�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
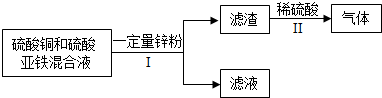
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
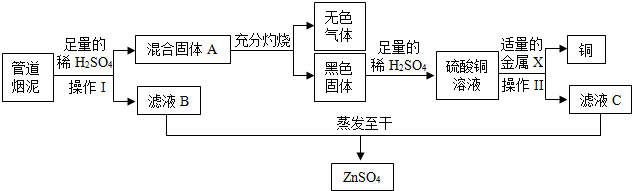
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��������ʰ����ʡ���������ᡢ��Ρ��л�����࣬ͼ�мס��ҡ��������ֲ�ͬ�������ʣ�����������֮����ܷ�����ѧ��Ӧ������˵��������ͼʾҪ����ǣ�������
��������ʰ����ʡ���������ᡢ��Ρ��л�����࣬ͼ�мס��ҡ��������ֲ�ͬ�������ʣ�����������֮����ܷ�����ѧ��Ӧ������˵��������ͼʾҪ����ǣ�������| A������Ϊ�����ʱ��X����Ϊ�������� |
| B�����ס��ҡ����ֱ�Ϊ�ᡢ���ʱ��X����Ϊ̼���� |
| C����XΪ����ʱ���ס��ҡ�������Ϊ���������ס������� |
| D����XΪϡ����ʱ���ס��ҡ�������Ϊ����ͭ��������ͭ�������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��22�춬������ƥ���˶��ᣬ��2014��2��7����23���ڶ���˹���������о��У��һ���ϩ����һ�ֲ����˶�Ա���õ��˷ܼ����仯ѧʽΪC20H32O���һ���ϩ����
��22�춬������ƥ���˶��ᣬ��2014��2��7����23���ڶ���˹���������о��У��һ���ϩ����һ�ֲ����˶�Ա���õ��˷ܼ����仯ѧʽΪC20H32O���һ���ϩ�����鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com