
����к�Ρ��Ļ�ѧʽΪMgSO4��KCl��xH2O����һ����ȡ�طʵ���Ҫԭ�ϣ�������ˮ�õ�KCl��MgSO4�Ļ����Һ��ij��ѧ�С��Ϊ�˲ⶨ����к�Ρ���KCl�������������������������ʵ�鷽����

�Իش��������⣺
(1)����Ϊ���� �ȽϺ����������� ��
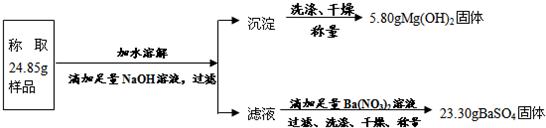
(2)��ѡ�����������е����ݣ�����24.85g��к����Ʒ��MgSO4��������(д���������) ����
(3)��ѡ�����������е����ݣ�����24.85g��к����Ʒ��KCl������������(д���������) ��
��
(1) �� (1��)
���� �������������ʶ��ܲⶨ��������һֻ�ܲⶨ����þ���ܲⶨ�Ȼ��ء���1�֣�
��(2) �⣺�����Ʒ��MgSO4������ΪX
����������MgSO4+Ba(NO3)2��BaSO4��+Mg(NO3)2���������������������� ��1�֣�
���������� 120 233
X 23.30g
120:233��X:23.30g������������������������������������ ��1�֣�
X=12.00g
���� �𣺸���Ʒ��MgSO4������Ϊ12.00g ��1�֣�
��(3) �⣺�����Ʒ��KCl������ΪY
�������� AgNO3+KCl��AgCl��+KNO3 ��1�֣�
74.5 143.5
Y 14.35g
74.5:143.5��Y:14.35g ��1�֣�
Y��7.45g
����Ʒ��KCl������������7.45g/24.85g��100%��29.98% ��1�֣�
�𣺸���Ʒ��KCl����������Ϊ29.98%
˵�����������������ⷨҲ��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�꽭��ʡ�����й������п���ѧ��ģ�Ծ��������棩 ���ͣ�ѡ����
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com