
��ҵ�ϣ�ͨ������ת�����Ƶ�KClO3���塣

��1��KClO3����Ԫ�صĻ��ϼ�Ϊ_______��
��2������������NaCl��Һ�ɴ���ˮ���ƶ��ɣ�����ʱ��ȥ����ˮ����ɳ�����õIJ�����_______��
��3�����Ƣ��з�Ӧ�Ļ�ѧ����ʽ��NaCl+3H2O=NaClO3 +3_______
��4����֪NaClO3+KCl=NaCl+KClO3������������������Һ��KClO3��_______���������Һ����������Һ������
��5�����������У���ѭ�����õ�������_______
 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2017�콭��ʡ�Ž�����������Ƭ�����꼶��ѧ�ڵ�һ��������ѧ�Ծ��������棩 ���ͣ�ѡ�������
�������ʱ��ֵ����ʣ������������ʵ��ǣ�������
A. þ��ȼ�� B. ���ܱ������ C. ˮ�ܱ�ɱ� D. ̿����������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017��ɽ��ʡ����в���ѧУ���꼶��3�£��п�ģ�⻯ѧ�Ծ��������棩 ���ͣ�ѡ�������
�������ʵ���������;�Ķ�Ӧ��ϵ������ǣ�������
ѡ�� | ���� | ��; |
A | �������Ƴʼ��� | ������������ |
B | Ũ���������ˮ�� | �������� |
C | ϡ��������ijЩ���������ﷴӦ | ������ |
D | ������������ijЩ�ǽ��������ﷴӦ | ���ն������� |
A. A B. B C. C D. D
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�츣��ʡ���꼶��ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ�ѡ�������
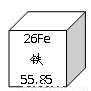
��ͼΪԪ�����ڱ���4���ڵ�һ���֡��ݴ��ж�����˵���д������( )
26 Fe �� 55.85 | 27 Co �� 58.93 | 28 Ni �� 58.69 | 29 Cu ͭ 63.55 |
A. ��Ԫ�صķ���ΪN i B. ��Ԫ�ص����ԭ��������58.93g
C. ��Ԫ�ض����ڽ���Ԫ�� D. �����Ҹ�Ԫ�ص�ԭ��������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�챱���в�ƽ�����꼶��ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ�̽����
ijͬѧ������ͼ��ʾװ�ý���ʵ�顣��֪�������Ż����40��
����I.���װ�õ������ԡ�
����II.��ʢ���������Ĵ���ȼ�ճ�����Aƿ�У���ƿ�м���80����ˮ������ƿ����
����III.��K1��K2����a����ƿ�й����������ƿ�е�Һ�����ȼ�ճײ�ʱ���ر�K1��K2����ʱ��Ͳ��ˮ�����Ϊ200mL��
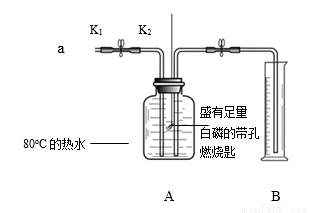
��1��������У��ر�K1��K2��۲쵽��������______����Ӧ�Ļ�ѧ����ʽΪ______��
��2���ԱȲ���II��III��֪����ȼ��ȼ�յ�����֮һ��______��
��3����װ����ȴ�����º�K2���۲쵽______��˵�������������ĺ���Լռ1/5��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�챱���в�ƽ�����꼶��ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ���Ϣ������
��������Ԫ����ɵġ�
��1��Ԫ�����ڱ�����Ԫ�ص���Ϣ��ͼ��ʾ����ԭ�ӵ����ԭ������Ϊ_____��
��2��O2��O3������Ԫ����ɵ����ֵ��ʣ��������ǵ������кܴ�IJ��죬��ԭ����_____ ��
��3��ij�л����ڴ�������ȫȼ��ֻ����CO2��H2O������л���������һ�����е�Ԫ����_____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�챱���в�ƽ�����꼶��ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ�ѡ�������
�����ӻ�������ָ�ڷ��ӳߴ��������һ������������䳤�̽��൱��l�������ң�1����=10-9�ף�������������ʽ��ͨ���ⲿ�̼�������ܡ����յȣ�ʹ���ӽṹ�����ı䣬�Ӷ��������������й��ڷ��ӻ�����˵���У���ȷ����
A. ������ֱ�ӹ۲쵽���ӻ���
B. ���ӻ������������У������˻�ѧ�仯
C. ���ӻ�������������Ҫ����
D. ���ӻ�������������������ʽ����ͨ��������ͬ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�챱���в�ƽ�����꼶��ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ�ѡ�������
ͨ��ʵ��ⶨ�˿�������ɵĿ�ѧ����
A. �Ž��з� B. ������ C. ������ D. ţ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ����ȫ����ǿУ��2016���Ĵ�ʡ���꼶�п���ϰ��ѧ�Ծ��������棩 ���ͣ�ѡ����
����ʵ�����������ȷ����
A����ͭ���еμ�ϡ���ᣬ��������ɫ����
B���Ȼ�����Һ��ϡ�����ϲ�����ɫ����
C����˿��������ȼ�ղ�����������
D����������ʪ��ĺ�ɫʯ����ֽ����ֽ����
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com