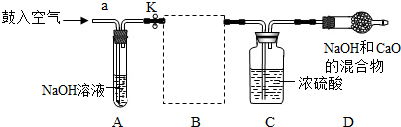
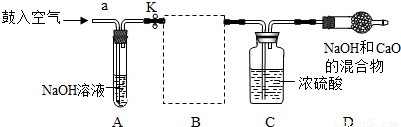
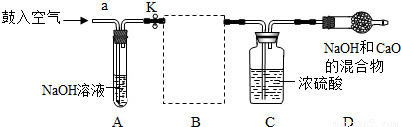
�⣺��1�������������տ����ж�����̼����ˮ��̼���ƶ����ʣ�
�ʴ�Ϊ��2NaOH+CO
2�TNa
2CO
3+H
2O��
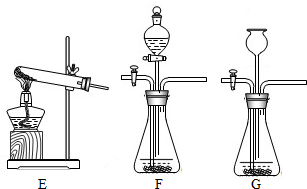
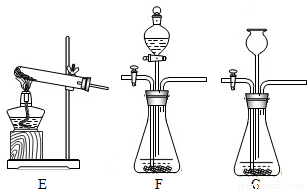
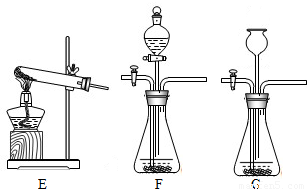
��2��װ��E��������ϡ�����������Ʒ�ķ�Ӧ��װ��G����©��δ����Һ�����£���ʹ����������ӳ���©���ݳ�����װ��E��G��������B����װ�ã�
�ʴ�Ϊ��F��
��3��̼���������ᷢ�����ֽⷴӦ�����������ơ�ˮ�Ͷ�����̼��
�ʴ�Ϊ��Na
2CO
3+H
2SO
4�TNa
2SO
4+H
2O+CO
2����
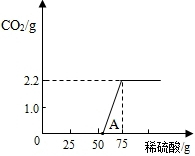
��4����μӷ�Ӧ��̼�������Ϊx
Na
2CO
3+H
2SO
4�TNa
2SO
4+H
2O+CO
2��
106 44
x 2.2g
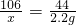
x=5.3g
��Ʒ���������Ƶ���������=
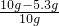
��100%=47%
�ʴ�Ϊ��47%��
��5��װ��C�е�Ũ���������ջ��ڶ�����̼�����е�ˮ���õ�����Ķ�����̼���壬���������л��е�ˮ�Բⶨ�����Ӱ�죻
�ʴ�Ϊ�����ջ��������е�ˮ����������壩����ֹ�����е�ˮ�Բⶨ������Ӱ�죻
��6��ʵ��ǰ����Ŀ����еĶ�����̼��װ��A�������������գ�ʣ��������װ���ڵĿ����ų�������װ���ڿ����ж�����̼�Բⶨ�����Ӱ�죻��Ӧ�����������������װ���ڲ����Ķ�����̼���岻�ܱ�װ��Dȫ�����գ���ɶ�����̼����ƫС��ʹ�ü��������Ʒ��̼��������ҲƫС����������ⶨ���������ƵĽ��ƫ��
�ʴ�Ϊ��CO
2��ƫ��
��������1���������ƻ����տ����ж�����̼���̼���ƶ����ʣ�
��2��������Ʒ��ϡ���ᷴӦ����Ҫ���ȣ����Բ���ҪѡEװ�ã���װ��G�������Բ��ã�ʹ�����������ݳ���Ҳ����ѡGװ�ã�
��3��̼����������Ӵ�ʱ��ͨ�������ɷֶ�������Ӧ���ɷų����������̼��
��4��װ��D����������Ϊ̼���������ᷴӦ�ų��Ķ�����̼�������ɷ�Ӧ���ɶ�����̼�����������ݷ�Ӧ�Ļ�ѧ����ʽ������μӷ�Ӧ��̼���Ƶ���������Ʒ������̼�����������Ʒ��������������������������������Ʒ�����ȿɼ�����Ʒ���������Ƶ�����������
��5��Ũ���������ˮ�ԣ������ջ��ڶ�����̼�����е�ˮ�֣����õ�����Ķ�����̼���壻
��6�����ó�ȥ������̼�Ŀ����ų�װ���ڲ�����������������ж�����̼��ʵ��������Ӱ�죻��Ӧ�����������ɰѲ�����װ���ڵĶ�����̼����ȫ�����գ�ʹ�ⶨ�����ȷ��
��������������ƫ�����ʱҪע�⣺������̼������ƫС��ᵼ��̼���Ƶ�����ƫС�������ղⶨ������Ʒ���������Ƶ������������������������Ĵ�С�����������̼������С�෴��



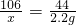 x=5.3g
x=5.3g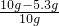 ��100%=47%
��100%=47%


 ijУ�о���ѧϰС�������һ����Ȥ��ʵ��̽����
ijУ�о���ѧϰС�������һ����Ȥ��ʵ��̽����