
 ����������ԭ����ͭʵ��ⶨˮ�������ͼ1����ش��������⣺
����������ԭ����ͭʵ��ⶨˮ�������ͼ1����ش��������⣺| ʵ��ǰ | ʵ��� | |
| ����ͭ�Ͳ����ܵ������� | 65.6g | 59.2g |
| ��������U�ܵ������� | 100.8g | 108.0g |
| 65.6g-59.2g |
| (108.0-100.8)-(65.6g-59.2g) |
| 6.4g |
| 0.8g |
| 8 |
| 1 |
| 65.6g-59.2g |
| (108.0-100.8)-(65.6g-59.2g) |
| 6.4g |
| 0.8g |
| 8 |
| 1 |
| 65.6g-59.2g |
| (108.0-100.8)-(65.6g-59.2g) |
| 6.4g |
| 0.8g |
| 8 |
| 1 |
| 65.6g-59.2g |
| (108.0-100.8)-(65.6g-59.2g) |
| 6.4g |
| 0.8g |
| 8 |
| 1 |


 ѧҵ����һ��һ��ϵ�д�
ѧҵ����һ��һ��ϵ�д� Сѧ��ʱ��ҵȫͨ����ϵ�д�
Сѧ��ʱ��ҵȫͨ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
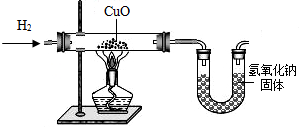
����������ԭ����ͭʵ��ⶨˮ����ɣ���ͼ��ʾ������ش��������⣺

��1��ʵ���У���п����ϡ���ᷴӦ��ȡ������ʵ�鿪ʼǰ��Ҫ�ȼ���______��Ȼ��Ӧ��ͨһ��ʱ���������ټ�������ͭ���з�Ӧ������ҪĿ����______��д���÷�Ӧ�Ļ�ѧ����ʽ______��
��2�������е�װ�ò�ã�ˮ���⡢��Ԫ�ص������ȴ���1��8�����ָý���Ŀ��ܵ�ԭ������Щ��______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ����������ģ ���ͣ��ʴ���
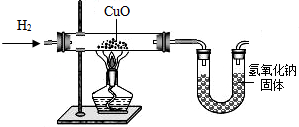
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010���Ϻ��л������п���ѧ��ģ�Ծ��������棩 ���ͣ������
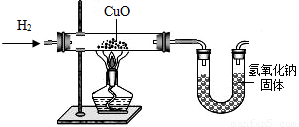
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com