
����Ŀ��1926�꣬�ҹ�������ѧ�Һ�°����������˺����Ƽ���ٽ����ҹ����幤ҵ�ķ�չ�������Ƽ���Ľ����������������������з�Ӧ��
��NaCl��NH3��CO2��H2O===NaHCO3��NH4Cl
��2NaHCO3![]() Na2CO3��H2O��CO2��
Na2CO3��H2O��CO2��
��1�������Ƽ���Ƶ���������ָ________��
��2����ҵ���������У�����ˮ���ն�����̼������̼�����ƺ��Ȼ�泥��ڳ����£��������ȴ���Һ�нᾧ��������________�����������������塣
��3������Na2CO3��NaCl�Ļ������Ʒ22.3 g���������ɾ����ձ��У���һ��������ˮʹ����ȫ�ܽ⡣��������Һ����μ���������������Ϊ7.3%��ϡ���ᣬ�ձ�����Һ�����������ϡ�����������ϵ������ͼ��ʾ���Իش��������⣺
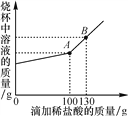
�ٵ���������ϡ������ͼ��B��ʱ���ձ�����Һ�������Ϊ________��д��ѧʽ����
����Na2CO3��NaCl�Ļ������Ʒ�У���Na2CO3������Ϊ________g��
�۵���������ϡ������ͼ��A��ʱ����ͨ�����㣬����¶�ʱ���ò�������Һ�����ʵ�����________����������ȷ��0.1 g����
���𰸡� ̼���� ̼������ NaCl��HCl 10.6 23.4 g
����������1�������Ƽ���Ƶ���������ָ����----̼���ơ�
��2���ڳ����£�̼�����Ƶ��ܽ��С���Ȼ�淋��ܽ�ȡ�
��3���ٵ���������ϡ������ͼ��B��ʱ��ϡ����ʣ�࣬�ձ�����Һ�����ɵ��Ȼ�����Һ��ʣ���ϡ���ᡣ
�ڵ���������ϡ������ͼ��A��ʱ��̼������ϡ����ǡ�÷�Ӧ����������̼���Ƶ�������x��
Na2CO3 + 2HCl == 2NaCl + H2O + CO2��
106 73
x 100g��7.3%
![]() =
=![]() �����x=10.6g
�����x=10.6g
���赱��������ϡ������ͼ��A��ʱ��̼������ϡ���ᷴӦ�����Ȼ��Ƶ�������y��
Na2CO3 + 2HCl == 2NaCl + H2O + CO2��
106 117
10.6g y
![]() =
=![]() �����y=11.7g
�����y=11.7g
ԭ��������Ȼ��Ƶ�����Ϊ��22.3 g-10.6 g=11.7g
A��ʱ��Һ���Ȼ��Ƶ�����Ϊ��11.7g+11.7g=23.4g



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������������������һ����ʳ����Һ����ȷ�IJ���˳����
A�����㡢�������ܽ⡢�����Լ�ƿ�����ϱ�ǩ
B���ܽ⡢���㡢�����������Լ�ƿ�����ϱ�ǩ
C���������ܽ⡢���㡢�����Լ�ƿ�����ϱ�ǩ
D�����������㡢�ܽ⡢�����Լ�ƿ�����ϱ�ǩ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����б仯���ڻ�ѧ�仯����
A������������� B����ѩ�������� C����ƹ���˸ D����ֽ�۳�ֽ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��1926�꣬�ҹ�������ѧ�Һ�°����������˺����Ƽ���ٽ����ҹ����幤ҵ�ķ�չ�������Ƽ���Ľ����������������������з�Ӧ��
��NaCl��NH3��CO2��H2O===NaHCO3��NH4Cl
��2NaHCO3![]() Na2CO3��H2O��CO2��
Na2CO3��H2O��CO2��
��1�������Ƽ���Ƶ���������ָ________��
��2����ҵ���������У�����ˮ���ն�����̼������̼�����ƺ��Ȼ�泥��ڳ����£��������ȴ���Һ�нᾧ��������________�����������������塣
��3������Na2CO3��NaCl�Ļ������Ʒ22.3 g���������ɾ����ձ��У���һ��������ˮʹ����ȫ�ܽ⡣��������Һ����μ���������������Ϊ7.3%��ϡ���ᣬ�ձ�����Һ�����������ϡ�����������ϵ������ͼ��ʾ���Իش��������⣺

�ٵ���������ϡ������ͼ��B��ʱ���ձ�����Һ�������Ϊ________��д��ѧʽ����
����Na2CO3��NaCl�Ļ������Ʒ�У���Na2CO3������Ϊ________g��
�۵���������ϡ������ͼ��A��ʱ����ͨ�����㣬����¶�ʱ���ò�������Һ�����ʵ�����________����������ȷ��0.1 g����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������ϵ��й����������ϵ��Ȳ��ó����ٴγ�Ϊ����ʩչ���յ���̨���������ʦ�и���������:����ʱ���ּ��Ͼ��ּӴף���ʹ�˱�������ɿڣ�ԭ���Ǵ��е��������Ͼ��е��Ҵ����������������±����Ǽ��ֳ���������������������⣺
�������� | ������� | �������� | ������� | �������� |
��ѧʽ | C2H4O2 | C3H6O2 | C3H6O2 | X |
��1���������(C2H4O2)��̼Ԫ�ء���Ԫ�ء���Ԫ�ص�������Ϊ _______��
��2����������(C3H6O2)��̼Ԫ�ص���������Ϊ_______(��������ȷ��0.1%)��
��3���ȽϹ�����ѧϰ��ѧ����Ҫ�������ݱ��Ʋ�X�Ļ�ѧʽΪ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij��ѧ��ȤС�����ⶨij���ۻ�����������������������ǽ�������ͼ��ʾ��ʵ�顣����㣺
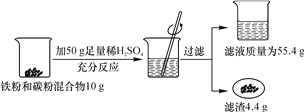
��1�������������������
��2����Ӧ���ձ���ϡ������ʣ�࣬���������ϡ���������ʵ�����������
��3������98%��Ũ�������Ƹ�Ũ�ȵ�ϡ����200 g��Ҫˮ���ٿˣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����Դ�뻷���ѳ�Ϊ���������ע�����⡣
(1)Ŀǰ��������������Դ��ú��________����Ȼ���Ȼ�ʯȼ�ϡ�
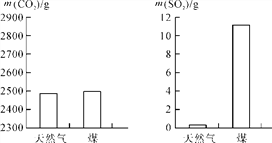
(2)���ȼ��1 000 g��Ȼ����ú��������CO2��SO2�������������ͼ��ʾ������ͼʾ����������˵����ȷ����________��
A��úȼ�ղ������������������
B������Ȼ���в�����Ԫ��
C��úȼ�նԻ���Ӱ���С
D��ú����Ȼ����ȼ�ն��������������
(3)�����������úͿ���������Դ����̫���ܡ�________��________�ȡ�
(4)�Ͼ���»���ʹ�õ�ȼ��ΪA���ʣ�������A��B�����г��ȼ�գ�������Ӧ��A��5B![]() 3C��4D(������ʾ��ͼ������ʾ)��
3C��4D(������ʾ��ͼ������ʾ)��
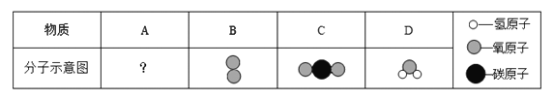
����������Ӧ���ṩ�������������Ե�����Ϊ____________��
��A�����и�Ԫ�ص�������Ϊ________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������ʵ˵�������к��еijɷ֣�ͨ�����ֵ��ܻᷢ����ͬ��ɫ�Ĺ⣬˵�������к���____________��������ռ�������������_________���ؿ��к������Ľ���Ԫ����_______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����������ʵ����������ڸ����ʵĻ�ѧ���ʵ����������������������� �� ��
A. ͭ�ĵ����� B. Һ���ķе�� C. �����ƵĻ����� D. �����ܶ�С
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com