

���� ��1������ÿ��ˮ������2����ԭ�Ӻ�1����ԭ�ӹ��ɽ��з�����
��2�����ݻ�ѧʽ��������з�����
��3�����ݷ��ӵĸ���ͷ��ӵĻ����������з�����
��4��������Һ�Ǿ�һ���ȶ��Ļ������з�����
��5������Ӳˮ�����ķ�������������Ӳˮ��ˮ�����ַ���������
��6�����ݺ�������ˮ��Դ�ͷ�ֹ������Ⱦ������з������жϣ��Ӷ��ó���ȷ�Ľ��ۣ�
��7���ٸ���ˮ�ֽ�������������������з�����
�ڸ�����ʯ����ˮ��Ӧ�����������ƽ��з�����
��� �⣺��1��ÿ��ˮ������2����ԭ�Ӻ�1����ԭ�ӹ��ɣ�A���Ա�ʾ����ˮ�����ӣ�
��2��H2O�Ӻ���Ͽ��ɱ�ʾˮ�����ɱ�ʾˮ��Ԫ����ɣ���ˮ������Ԫ�غ���Ԫ����ɵģ�
��3��A����ˮ���������У��¶����ߣ�ˮ����֮�������A����
B�����������ڲ��ϵ��˶��ģ���ˮ���������У��¶����ߣ������˶������ʸ��죬��B��ȷ��
C��ˮ���������������仯�����ӱ���û�з����ı䣬��C����
D��ˮ������ˮ���ӻ���������˶��ٶȼӿ죬ˮ����֮��ļ�����ˮ���ӵ�������䣬��D����
��4���ƾ������������ص�����������ˮ����ˮ���γɾ�һ���ȶ��Ļ��������γ���Һ�������Ͳ�����ˮ�����ܺ�ˮ�γɾ�һ���ȶ��Ļ����������γ���Һ��
��5��������п�ʹˮ�и�þ���ӻ�����ת��Ϊ�������Ӷ�����ˮ��Ӳ�ȣ�Ӳˮ�м������ˮ������ĭ�٣���ˮ�м������ˮ������ĭ�࣬�ʷ���ˮ������Ӳˮ����ˮ��
��6��A������ʹ�û���ũҩ����Ⱦ������ʹˮ�ܵ���Ⱦ�������ڱ���ˮ��Դ����A����
B�����Ͼɵ�����������л���Ⱦ�����͵���ˮ��Դ����B����
C��������ֳ����ˮֱ�����뽭�ӻ���Ⱦˮ��Դ����C����
D����ˮ��Դ��������ֲ���������ڱ���ˮ��Դ���������ˮ����Ⱦ����D��ȷ��
��7����ˮ��ͨ���������������������������ѧ��Ӧ����ʽ��2H2O$\frac{\underline{\;ͨ��\;}}{\;}$2H2��+O2����
����ʯ����ˮ��Ӧ�����������ƣ���Ӧ�Ļ�ѧ����ʽΪ��CaO+H2O�TCa��OH��2��
�ʴ�Ϊ����1��A����2����Ԫ�غ���Ԫ�أ���3��B����4��C����5��������У�����ˮ����6��D����7����2H2O$\frac{\underline{\;ͨ��\;}}{\;}$2H2��+O2������CaO+H2O=Ca��OH��2��
���� ��ѧ��Դ���������Ҳ������������������������������������صĻ�ѧ֪ʶҲ����Ҫ���п��ȵ�֮һ��


 �п�������㾫��ϵ�д�
�п�������㾫��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ��̽����
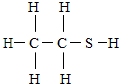 ������2000��漪��˹�����¼����¼����������ʣ������н�������ڷ�֮һ������ʱ�����ζ�Ϳ��ᵽ��ͨ��������Һ��ú��������ζָʾ�������Ľṹʽ��ͼ����ش��������⣺
������2000��漪��˹�����¼����¼����������ʣ������н�������ڷ�֮һ������ʱ�����ζ�Ϳ��ᵽ��ͨ��������Һ��ú��������ζָʾ�������Ľṹʽ��ͼ����ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ������ճ������ũҵ�����벻��ˮ����Һ����ش�
������ճ������ũҵ�����벻��ˮ����Һ����ش��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ʵ��Ŀ�� | ����1 | ����2 | |
| A | ��ȥͭ���е����� | �ô������� | ����AgNO3��Һ������ |
| B | �������������¯������ | �����̪��Һ���۲����� | ���봿��۲����� |
| C | ���������̼������ | ͨ��CaCl2��Һ�У��۲� | ����ȼ�ŵ�ľ�� |
| D | ��������������Һ���ֱ��� | �μ���ɫ��̪��Һ���۲� | ����̼������Һ���۲����� |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� |  ��ȼ�ƾ��� | B�� | 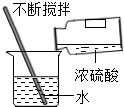 ϡ��Ũ���� | C�� |  ���������� | D�� |  ��������ζ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com