
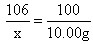 ��x=10.6g
��x=10.6g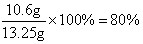
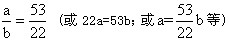
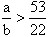 ��
��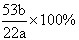 (�������T����)
(�������T����)

 �������Ӧ���⼯ѵϵ�д�
�������Ӧ���⼯ѵϵ�д� �ۺ��Բ�ϵ�д�
�ۺ��Բ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| +4 |
| C |
| +4 |
| C |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| +4 |
| S |
| +4 |
| S |
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| +4 |
| C |
| +4 |
| C |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ͬѧ����ѧϰ���εĻ�ѧ�����Ժ�չ�˲ⶨ̼���ƺ��Ȼ��ƵĹ���������̼���Ƶ�����������̽��ʵ�飬�������������ʵ�鷽��
 ������һ������ת����
������һ������ת����


ͼ1 ͼ2 ͼ3
��1�� ����ͼ1��ʾ����wg�Ļ����������ϡ���ᷴӦ�ⶨ������CO2�����������װ�������Եķ�����
��2�� ��ͬѧ�����ͼ2����ͼ1�е��ռ�װ�ã��������CO2��������
����ƫ��ƫС�䣩������ ����ĸĽ������� �����ƿ��ԭ�еĿ�����ʵ����___________(��С���û�С�)Ӱ�죬������øĽ����װ�ò��CO2��������ΪVml (��ʱCO2���ܶ�ΪPg/ml)��ԭ�������Na2CO3�����������ļ���ʽΪ_________________
(3)Ҳ������ͼ3װ�òⶨCO2������(��ʯ�ҵijɷ���CaO��NaOH�Ļ����)��ͼ3ʵ��װ������Ҫ������Щȱ�ݣ���Щȱ�ݶ�ʵ�����к�Ӱ�죿
| ��Ҫȱ�� | ��ʵ������Ӱ�� |
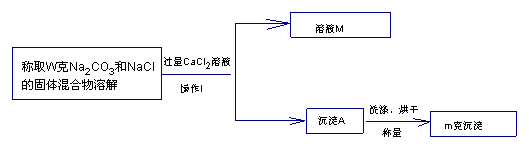 ����������������(��ͼ��ʾ)
����������������(��ͼ��ʾ)
(1)����A�Ļ�ѧʽ��_______________����ҺM�����ʵĻ�ѧʽ��_______________
(2)ȷ��CaCl2��Һ�Ƿ�����ķ�����_____________________________
(3)�ù���������Na2CO3�����������ļ���ʽΪ_________________________
(4)���²ⶨ���ƫ���ԭ�������_________________________
����������������

(1)������2����������______________
(2)����ʵ�����ݼ���ù���������Na2CO3����������Ϊ________(����һλС��)
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com