
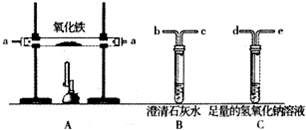
ijѧ���Ľ�һ����̼��ԭ��������ʵ��װ�ã���ͼ��ʾ��

��1�����ȸ����������ȣ���ͨ��һ����̼�����ܲ����ĺ����_____________________��
��2��д��A�з�����ѧ��Ӧ�ķ���ʽ��___________________________��
��3������Bװ������������������жϷ�Ӧ�Ƿ�ʼ��������B��ʢ�ŵ��Լ���_________����ѧ��Ӧ�ķ���ʽΪ____________________________________��
��4��Cװ�õ�������____________________________________��
��5��Cװ���ռ�����ķ�������___________________________���ռ���Cƿ�е�������δ���____________________________________��
��6��A��B����������ֱ���_____________________��_______________________��
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 25����ä���𱯾磡2008��12��1�գ�����ijСѧ��̿¯ֱ��ȼúȡů����������11��ѧ���ж�������
25����ä���𱯾磡2008��12��1�գ�����ijСѧ��̿¯ֱ��ȼúȡů����������11��ѧ���ж��������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
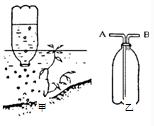 19���Ͼ������ķ羰��--����Ȫ��ˮ���д���������ð������һ�������飬����Ȫ�ɴ˵�����ijѧ���Ʋ�������������������������ϵ�֪�������к�60��70%��CH4��������CO2��N2��CO�ȣ�������ʵ���������Ȫð���������ɣ����������ͼ����ʾ��ȡ�����������յ�ѩ����ˮ����ƿװ��ˮ��������Ȫˮ�У�ƿ�ڶ�ˮ��ð�������ݽ����ռ���
19���Ͼ������ķ羰��--����Ȫ��ˮ���д���������ð������һ�������飬����Ȫ�ɴ˵�����ijѧ���Ʋ�������������������������ϵ�֪�������к�60��70%��CH4��������CO2��N2��CO�ȣ�������ʵ���������Ȫð���������ɣ����������ͼ����ʾ��ȡ�����������յ�ѩ����ˮ����ƿװ��ˮ��������Ȫˮ�У�ƿ�ڶ�ˮ��ð�������ݽ����ռ����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����

| ||
| ||
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com