
 ��ͼ�dz����������ʵ��ܽ�����ߣ�����ͼʾ�ش�
��ͼ�dz����������ʵ��ܽ�����ߣ�����ͼʾ�ش����� ��1���ܽ�����ߵĽ����ʾ���¶��������ʵ��ܽ����ȣ�
��2������t1��ʱA���ܽ�Ⱥͱ�����Һ��Ϊ��������Һ�ķ������ǣ�
��3�����¶Ȳ������ʵ��ܽ�Ȳ��䣬�������Ϣ�������
��4����AB���ܽ�����¶�Ӱ��������ɷ����ᴿ���ʵķ�����
��� �⣺��1��������M���ʾ����t2��ʱA��C�����ʵ��ܽ�ȵĹ�ϵΪA����C��A��B��������t3��ʱ�ܽ�ȵ�A����B��
��2��t1��ʱ��A���ܽ����40g����100gˮ������ܽ�40g��A����˽�10gA���ʷ���100gˮ�У�����ܽ�����õ���Һ�Dz�������Һ����ʹt2��ʱ��C���ʵı�����Һ��Ϊ��������Һ������C���ܽ�����¶����߶����ͣ����Կɲ�ȡ�ķ����ǣ���ˮ���£�
��3��30��ʱA�IJ�������Һ���ڸò�������Һ�м�A�����ͣ������ʵ���������Һ���������ӣ�����Һ��ˮ���������䣬�¶Ȳ��䣬30��ʱA���ܽ�Ȳ��䣻
��4��t3��ʱA�ı�����Һ�к���������B����Ҫ�ᴿA���ɲ��ý��½ᾧ�ķ�������ΪA���ܽ�����¶ȵ�Ӱ���B��
�ʴ�Ϊ����1��=��������2�������ͣ���ˮ�����£�����3���٢ڣ���4�����£�
���� �����ؼ���Ҫ��Ϥ�ܽ�����ߵ����壬֪�����ߵĽ����ʾ�����壬������Һ�벻������Һ���ת�����ᴿ���ʵķ�����֪ʶ�����ܽ�����������


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ˮ������֮Դ������֮���������౦�����Ȼ��Դ��
ˮ������֮Դ������֮���������౦�����Ȼ��Դ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ��̽����
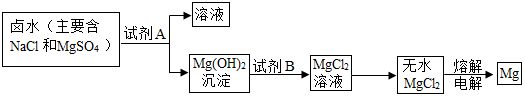
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ɽ��ֽ | B�� | ��ɽ�մ� | C�� | ʯ���ۻ� | D�� | �ƾ��ӷ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���� | B�� | ���� | C�� | �� | D�� | ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
 ��ͼ��ij���ӵĽṹʾ��ͼ������˵����ȷ���ǣ�������
��ͼ��ij���ӵĽṹʾ��ͼ������˵����ȷ���ǣ�������| A�� | �����Ӹ����Ӳ���ӵ�������ͬ | B�� | �����Ӻ����������Ϊ8 | ||
| C�� | �����ӱ�ʾ����ԭ�� | D�� | ���������ڽ���Ԫ�� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com