����⣺�⣺��6���ٸ��ݲ������Ͽ�֪���������漰�ķ�Ӧ������Ϊ���£����ƾ��ƵĻ�����¶ȴﲻ�������������˻�ø���Ҫѡ�ƾ���ƣ�
����Ϊ��Ӧ����̼�����ڼ��������¿��ԺͿ����е�����������Ӧ���ɶ�����̼��Ӱ��ʵ����������Ŀ�ľ�������Թ��еĿ�����
����3.2gFe
2O
3��̿�۷�����Ӧ����C0
2������Ϊx��
2Fe
20
3+3C
4Fe+3C0
2��
320 132
3.2g x
=��ã�x=1.32g
��3.20g��������ȫ��Ӧʱ����CO
2������Ϊ1.32�ˣ�
��CO
2��C��0������=12��16��2=1 2��32=3��8��
�ݸ��������غ㶨�ɿ�֪��ʵ���������������Ϊ��48.48g+3.2g+2g��-52.24 g=1.44g��
��ʵ����ʵ�ʻ�����������Ϊ1.44�ˣ�
��7�����ۣ���Ϊ���ɵ���������Ϊ1.44 g������1.32g����������ﲻ��ȫΪCO
2��ԭ���費������
���ߣ�ʵ���������Ԫ������Ϊ0.96 g
ʵ�������̼Ԫ������Ϊ1.44 g-0.96 g=0.48 g
ʵ�������̼Ԫ������Ԫ��������=1��2
��Ϊ������̼Ԫ������Ԫ��������Ϊ1��2����������CO
2����Ԫ��������Ϊ3��8���ʲ��ﲻȫ��CO
2�����м����Ǵ���ģ�
�ʴ�Ϊ��
��6�����������
����Ϊ�þƾ���Ƽ��ȱȾƾ����ܻ�ø��ߵ��¶ȣ�
������Թ��еĿ���������������ֹ̼��������е�����������Ӧ��
��1.3g��
��CO
2��C��0������=12��16��2=1 2��32=3��8��
��1.44g��
��7�����ۣ���Ϊ���ɵ���������Ϊ1.44 g��Զ����1.32g����������ﲻ��ȫΪCO
2��ԭ���費��������
���ߣ�ʵ���������Ԫ������Ϊ0.96g��
ʵ�������̼Ԫ������Ϊ1.44 g-0.96 g=0.48g��
ʵ�������̼Ԫ������Ԫ��������=1��2��
��Ϊ������̼Ԫ������Ԫ��������Ϊ1��2����������CO
2����Ԫ��������Ϊ3��8���ʲ��ﲻȫ��CO
2�����м����Ǵ���ģ�

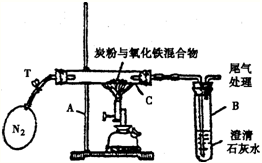 ��2005?��̶��ij��ѧ��ȤС���ѧ���о�������̼����Fe2O3�ڸ��������·�Ӧ���������ɷ֣�̽���������£�
��2005?��̶��ij��ѧ��ȤС���ѧ���о�������̼����Fe2O3�ڸ��������·�Ӧ���������ɷ֣�̽���������£�


 ��2005?��̶����ͼ���ñ���ģ������ʾij���ʷ�����ѧ�仯����ʾ��ͼ��ͼ�С�
��2005?��̶����ͼ���ñ���ģ������ʾij���ʷ�����ѧ�仯����ʾ��ͼ��ͼ�С� ���͡��ֱ��ʾ����Ԫ�ص�ԭ�ӣ����ø�ͼʽ��ʾ�Ļ�ѧ��Ӧ�ǣ�������
���͡��ֱ��ʾ����Ԫ�ص�ԭ�ӣ����ø�ͼʽ��ʾ�Ļ�ѧ��Ӧ�ǣ�������