�������̼����ɹ�Ρ�������ġ����ָNa
2CO
3�����Ρ���ָNaCl��
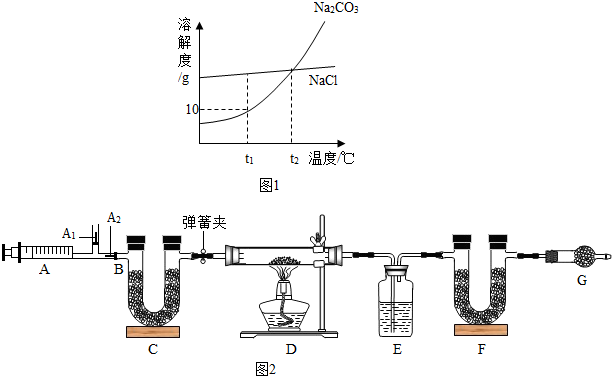
��һ��Na
2CO
3��NaCl���ܽ��������ͼ1��ʾ������ͼ�ش�
��t
1��ʱNa
2CO
3���ܽ��Ϊ
10
10
g��
t
2��ʱNa
2CO
3���ܽ��
�T
�T
NaCl���ܽ�ȣ������������������=����
�ڡ������̼��ԭ��������Na
2CO
3���ܽ�����¶Ƚ��Ͷ�
��С
��С
���������С�����䡱����
�ۡ�����ɹ�Ρ�������
A
A
������ţ��ķ�����ʹNaCl����������
A���紵��ɹ��ʹ�ܼ����� B�������¶ȣ�ʹNaCl�ܽ������
�������ҹ��ຣ�������õ�����Ȼ�����̼���Ƶľ��壬��ɿɱ�ʾΪaNa
2CO
3?bNaHCO
3?cH
2O��a��b��cΪ��������ȣ���ij��ѧ����С�����Ȼ��ijɷֽ���̽����
С��ͬѧΪ�ⶨ����ɣ���ȡ����Ȼ����Ʒ16.6g������ͼ2ʵ�飺
���������ϡ�
��1��̼���ƱȽ��ȶ�������ʱ���ֽ⣻
��2��2NaHCO
3 Na
2CO
3+CO
2��+H
2O ��3��ͼ��B��Ϊ����������ע����ʱA
1�رգ�A
2������ע����ʱ��A
1��������A
2�رգ�
��ʵ�鲽�衿
����װ��װ�ã���������� �ڷ�������ע���� �۳���E��F�������ܹرյ��ɼУ�����D���Թ�ֱ����Ӧ���ٽ��� �ݴ��ɼУ��ٴη�����������ע���� ���ٴγ���E��F��������
������̽����
��1��E�е�ҩƷΪ
Ũ����
Ũ����
��E��������
����ˮ����
����ˮ����
��
��2��C��F��G��װ�м�ʯ�ң�CaO��NaOH�Ĺ����������C��������
��ȥ�����еĶ�����̼��ˮ��������������
��ȥ�����еĶ�����̼��ˮ��������������
��F��������
�������ɵĶ�����̼
�������ɵĶ�����̼
��G��������
��ֹ�����еĶ�����̼��ˮ����������������뵽F�У�Ӱ�������̼�����IJⶨ
��ֹ�����еĶ�����̼��ˮ����������������뵽F�У�Ӱ�������̼�����IJⶨ
��
��3��ʵ�鲽�������ܷ�ߵ�
����
����
����ܡ����ܡ������������в���ݵIJ�����������õ�̼��������������
ƫС
ƫС
���ƫ����ƫС��������Ӱ�족�����ò�������ע����ʱ������Ŀ����
ʹ���ɵĶ�����̼��ˮ�������ճ��
ʹ���ɵĶ�����̼��ˮ�������ճ��
��4�����±���16.6g��Ȼ���нᾧˮ������Ϊ
1.8
1.8
g��Na
2CO
3������Ϊ
10.6
10.6
g������Ȼ��Ļ�ѧʽ��a��b��c=
2��1��2
2��1��2
��
| ��Ӧǰ |
��Ӧ�� |
| E������Ϊ100.0g |
E������Ϊ102.25g |
| F������Ϊ50.0g |
F������Ϊ51.1g |





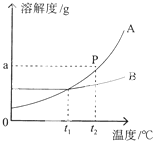 ��Һ�������Ϳ����о��й㷺����;�������ǵ������ܲ��ɷ֣�
��Һ�������Ϳ����о��й㷺����;�������ǵ������ܲ��ɷ֣� С��ͬѧ��������ͼ��ʾA��B���ֹ������ʵ��ܽ�����ߣ�
С��ͬѧ��������ͼ��ʾA��B���ֹ������ʵ��ܽ�����ߣ�