
| ��һ�� | �ڶ��� | ������ | ���Ĵ� | |
| ����ϡ��������� | 50g | 50g | 50g | 50g |
| ʣ���������� | 12.6g | 10.2g | 7.8g | 6.6g |
���� ͭ���ܺ�ϡ���ᷴӦ��þ�ܺ�ϡ���ᷴӦ��������þ���������ɱ������ݿ�֪����һ�Ρ��ڶ��κ͵������У�ϡ���ᶼ��ȫ��Ӧ�����Ĵ��У�ϡ����������ڶ��Ρ������γ�ַ�Ӧ����ٵ������������2.4g��˵�������ȡþ�ۺ�ͭ�ۻ���������Ϊ��12.6g+2.4g=15g��ͭ��������6.6g��
��� �⣺�ɱ������ݿ�֪����һ�Ρ��ڶ��κ͵������У�ϡ���ᶼ��ȫ��Ӧ�����Ĵ��У�ϡ����������ڶ��Ρ������γ�ַ�Ӧ����ٵ������������2.4g��˵�������ȡþ�ۺ�ͭ�ۻ���������Ϊ��12.6g+2.4g=15g��ͭ��������6.6g��
��1����ȫ��Ӧ����Һ�е����������ɵ�����þ��ʣ����ϡ���ᣬ���MgSO4��H2SO4��
��2��þ�ۺ�ͭ�۵Ļ������ͭ����������Ϊ��$\frac{6.6g}{15g}��100%=44%$�����44%��
��3�����������������Ϊx�����У�
Mg+H2SO4�TMgSO4+H2��
24 98
2.4g 50g��x
$\frac{24}{98}=\frac{2.4g}{50g��x}$
x=19.6%
������ϡ���������ʵ���������Ϊ19.6%��
���� �������ڼ����е�Ӧ�úܹ㷺�����Ĺؼ���Ҫ���������ʵ���������Ҫ���δ֪��֮��Ĺ�ϵ���ٸ��ݾ����������⣮



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | þ���ڿ�����ȼ�գ�����ҫ�۵İ⣬�ų����������ɺ�ɫ��ĩ״���� | |
| B�� | ��˿�������о���ȼ�գ��������䣬�ų����������ɺ�ɫ���� | |
| C�� | ľ̿��������ȼ�գ������⣬�ų�������������ʹ����ʯ��ˮ����ǵ����� | |
| D�� | �����ڿ�����ȼ�գ����������������ų����������ɰ�ɫ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��ͼΪ�ס������ֹ������ʵ��ܽ�����ߣ������ͼʾ�ش��������⣺
��ͼΪ�ס������ֹ������ʵ��ܽ�����ߣ������ͼʾ�ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������չ������ͨ���ᳫ��ɫ���� | |
| B�� | ���е�·ʹ��̫���ܾ��۵ƣ������ֻ��� | |
| C�� | �����Զ�Ǧ�ʣ�����ľ��Ǧ�� | |
| D�� | ���Ϊ�������ع���ת��Ϊ����ȼ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 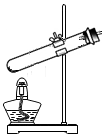 | B�� | 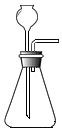 | C�� | 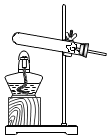 | D�� |  |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ���������θҺ��HCl��������Χ | 0.2g��0.4g |
| �û���θҺ��HCl������ | 1.495g |
| �û�������Ҫ��ȥ��HCl�������� | 1.095g |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com