�⣺��1���ɷ��ӵı�ʾ��������ȷ��д���ʵĻ�ѧʽ����ʾ����÷��ӣ������仯ѧʽǰ������Ӧ�����֣���2�������������ӿɱ�ʾΪ��2P
2O
5��
�����ӵı�ʾ�������ڱ�ʾ�����ӵ�Ԫ�ط������Ͻǣ���������������������������������ǰ�����������ں�1�����ʱ��1Ҫʡ�ԣ�����ʾ��������ӣ����������ӷ���ǰ������Ӧ�����֣���3���������ӡ�̼������ӷֱ�ɱ�ʾΪ��3Fe
2+��CO
32-��
��ԭ�ӵı�ʾ��������Ԫ�ط�������ʾһ��ԭ�ӣ���ʾ�����ԭ�ӣ�������Ԫ�ط���ǰ������Ӧ�����֣���һ����ԭ�ӱ�ʾΪ��O��
�ɻ��ϼ۵ı�ʾ���������仯ѧʽ��Ԫ�ص��Ϸ��������ź����ֱ�ʾ����������ǰ�������ں�ˮ����Ԫ����-2�ۿɱ�ʾΪ��H
2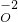
��
��2���ٸ���ij��Ȫˮ��ǩ�ϵ�һ���֣��������в��ֹ������ơ��ơ��ȡ���������Ԫ�أ�
��Ca
2+�еġ�2����ʾһ�������Ӵ���������λ������ɣ�
��4��������������ܹ��ɵĻ������У�NaCl��CaCl
2��Na
2SO
4��CaSO
4��
NaCl ����Է�������Ϊ��23+35.5=58.5
CaCl
2 ����Է�������Ϊ��40+35.5��2=111
Na
2SO
4 ����Է�������Ϊ��23��2+32+16��4=142
CaSO
4����Է�������Ϊ��40+32+16��4=136
�ɴ˿�֪����Է����������Ļ�����Ļ�ѧʽΪ��Na
2SO
4��
�ʴ�Ϊ����1��2P
2O
5��3Fe
2+��CO
32-��O��H
2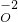
����2�����壻��һ�������Ӵ���������λ������ɣ��ۣ�Na
2SO
4��
��������1�����ӵı�ʾ��������ȷ��д���ʵĻ�ѧʽ����ʾ����÷��ӣ������仯ѧʽǰ������Ӧ�����֣�
���ӵı�ʾ�������ڱ�ʾ�����ӵ�Ԫ�ط������Ͻǣ���������������������������������ǰ�����������ں�1�����ʱ��1Ҫʡ�ԣ�����ʾ��������ӣ����������ӷ���ǰ������Ӧ�����֣�
ԭ�ӵı�ʾ��������Ԫ�ط�������ʾһ��ԭ�ӣ���ʾ�����ԭ�ӣ�������Ԫ�ط���ǰ������Ӧ�����֣�
���ϼ۵ı�ʾ���������仯ѧʽ��Ԫ�ص��Ϸ��������ź����ֱ�ʾ����������ǰ�������ں�
��2���ٸ���ij��Ȫˮ��ǩ�ϵ�һ���֣��ж�Ԫ�ص����࣮
�ڱ���Ԫ�ط������Ͻǵ����ֱ�ʾ���������������
�������������ӽ���ܽ�ϳɻ�����ֱ���㻯�������Է����������бȽϣ�
�����������ѶȲ�����Ҫ����ͬѧ�ǶԳ�����ѧ���ԭ�ӷ��š����ӷ��š����ϼۡ����ӷ��ŵȣ�����д������������

 ��1�����ʵ��Ļ�ѧ���ű�ʾ��
��1�����ʵ��Ļ�ѧ���ű�ʾ��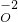 ��
��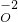 ����2�����壻��һ�������Ӵ���������λ������ɣ��ۣ�Na2SO4��
����2�����壻��һ�������Ӵ���������λ������ɣ��ۣ�Na2SO4��


 ��1�����ʵ��Ļ�ѧ���ű�ʾ��
��1�����ʵ��Ļ�ѧ���ű�ʾ�� ��1�����ʵ��Ļ�ѧ���ű�ʾ��
��1�����ʵ��Ļ�ѧ���ű�ʾ��