
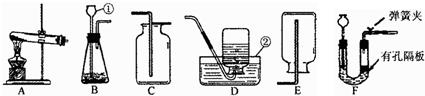
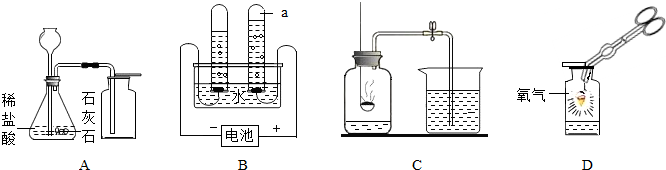
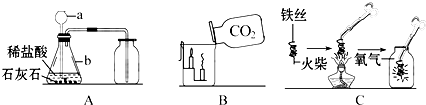
���������װ�ã��ش����⣺
��д����Ţ٢ڵ��������ƣ��� ���� ��
��ʵ������ȡH2����ѡ��װ�� ����дװ����ţ����ռ����ϴ�����H2��
���ж�CO2���ռ����ķ����� ��
���ø��������ȡO2��װ��A��������һ��Ķ��� ��
�ɶ�������غͶ������̵Ļ������ȡO2��Ĺ���������ٶ�����ȫ��Ӧ����ͨ�������IJ�ʵ������ɻ��ն������̡���ȷ�������Ⱥ�˳���� ����дѡ����ţ���
a����� b���ܽ� c������ d��ϴ��
�ʰѢ�����Һ�����ᾧ�ɵõ��Ȼ��ؾ��壬�����������в�������������
��
����װ��F��ȡ���壬�ڷ�Ӧ�����У��õ��ɼм�ס�������ϵ���Ƥ�ܣ���һ�����Ӧ�ͻ�ֹͣ����ԭ���� ��
 53���ò�ϵ�д�
53���ò�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ���� | þ�� | ��̼0.05%����˿ | ��̼0.2%����˿ | ��̼0.6%����˿ |
| ���� | ����ȼ�գ�����ҫ �۰⣬���� |
����ȼ�� ���ٻ��� |
����ȼ�� �������� |
����ȼ�գ��������� ����ȼ�գ��������� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com