��������Ҫ�Ľ������ϣ�
��1����������Ʒ����;�У����ý��������Ե���______������ĸ����ͬ����

��2�������й�������������ȷ����______
A������������ɫ���������ں�ɫ���� B���������������õ��ġ���������Ϊ���Ͻ�
C������������ȼ�����ɺ�ɫ�������� D���ؿ��е������Ի�������ʽ����
��3�����и�����Ʒ���õķ��ⷽ���ǣ�
������______����______���г�����______ˮ��ͷ______
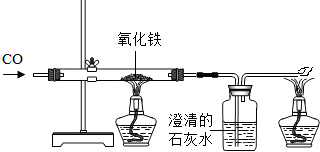
��4������ÿ�����Ȼ����ȡ�����Ľ�����������ȡ������������������¯�з����ķ�Ӧ�У���Fe
2O
3+3CO�T2Fe+3CO
2��C+O
2�TCO
2��CO
2+C�T2CO
��CaCO
3�TCaO+CO
2����CaO+SiO
2�TCaSiO
3�������Ϸ�Ӧ�ƶϣ�������ԭ����Ҫ��______��______��______��______
��������д��ţ���ԭ����ʯ�ķ�Ӧ��______�������ķ�Ӧ��______��������Դ�ķ�Ӧ��______��






 ��������Ҫ�Ľ������ϣ��ڽ��컴����ˮ�ɻ���ʱ�������˴����ĸ�����
��������Ҫ�Ľ������ϣ��ڽ��컴����ˮ�ɻ���ʱ�������˴����ĸ�����