
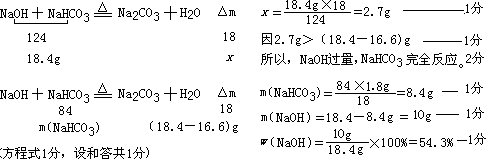



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
�¶� �ܽ�� �� |
0�� | 10�� | 20�� | 30�� | 40�� | 50�� | 60�� | 100�� |
| NaCl | 35.7 | 35.8 | 36.0 | 36.3 | 36.6 | 37.0 | 37.3 | 39.8 |
| NH4HCO3 | 11.9 | 15.8 | 21.0 | 27.0 | �� | -- | -- | -- |
| NaHCO3 | 6.9 | 8.1 | 9.6 | 11.1 | 12.7 | 14.5 | 16.4 | -- |
| NH4Cl | 29.4 | 33.3 | 37.2 | 41.4 | 45.8 | 50.4 | 55.3 | 77.3 |
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ʴ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010��ڶ�ʮ�조��ԭ����ȫ������ѧ����ѧ���ʺ�ʵ���������������������������Ծ��������棩 ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��1��ijС�մ���Һ�к�16.8gNaHCO3������һ�����ĵ��ʻ���X��ǡ��ʹ��Һ������ֻ��Na2CO3��������д��X�Ļ�ѧʽ��������
���� ��XΪNaOHʱ������Ϊ 8g ��
��XΪ ʱ������Ϊ ��
��XΪ ʱ������Ϊ ��
��XΪ ʱ������Ϊ ��
��2����������Ϊ18.4g��NaOH��NaHCO3�Ļ���װ��һ�ܱ������У���120����¶��½��м��ȣ�����ַ�Ӧ���ų�ʣ�����壬��ʱ�����ڹ������ʵ�����Ϊ16.6g���Լ���ԭ�������NaOH��NaHCO3��������Ϊ���٣�(��ʾ��NaHCO3+ NaOH120�� Na2CO3 +H2O)
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com