������㣬��ʱ�����ܷ⣮Ϊ�˽������������⣬����ˮ�м���������ƣ���ѧʽ��CaO2��������������ˮ��Ӧ�������������ƺ�������
��1��д������������ˮ��Ӧ�Ļ�ѧ����ʽ����ƽ��______
��2����ҵ����Ա����ͨ���������������ⶨ��������������Һ������������ʵ���������£���ȡ1.44g�������ƣ����뵽2000.00g��ˮ�У�������224mL���������������ܶ�Ϊ1.43g/L�����Լ���
�ٲ�����������g��
�������������ƶ���g��
����������������Һ�����������ٷֱ�Ũ�ȣ���
�⣺��1�����������Ϣ��֪������������ˮ��Ӧ�����������ƺ�����������ʽΪ��2CaO
2+2H
2O�T2Ca��OH��
2+O
2����
��2���⣺224ml��������Ϊ��1.43g/l��0.224l��0.32g����1.44gCaO
2��ˮ��Ӧ�������������Ƶ�������x
2CaO
2+2H
2O�T2Ca��OH��
2+O
2��
144 148
1.44g x
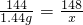
x=1.48g
224ml��������Ϊ��1.43g/l��0.224l��0.32g������������Һ������Ϊ1.44g+2000g-0.32g=2001.12g����������������Һ��������Ϊ
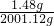
��100%=0.074%
�ʴ�Ϊ����1��2 CaO
2+2H
2O=2Ca��OH��
2+O
2��
��2���ٲ���������������0.32g��
����Һ���������Ƶ�����Ϊ1.48g��
����Һ���������Ƶ���������0.074%��
��������������ṩ����Ϣ����д���йط�Ӧ�Ļ�ѧ����ʽ����д��ѧ����ʽҪע�⣺�ٻ�ѧʽҪ��ȷ������ѭ�����غ㶨�ɣ���д��Ҫ�����������Ƿ��С�����������
����������������Ƶ������������ɵ�������������������Ӧ�����Һ���������÷�Ӧǰ����Һ��������������֮����ģ��ݴ˿���������Һ����������������
���������������غ㶨�������Ӧ������Һ���������ǽ��м���Ļ��������ֳ�����֪ʶ���������������

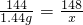
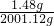 ��100%=0.074%
��100%=0.074%

 Ӯ�ڿ�����ʦ��ʱ�ƻ�ϵ�д�
Ӯ�ڿ�����ʦ��ʱ�ƻ�ϵ�д�