�⣺��1�����������غ㶨�ɺͻ�ѧ����ʽ�ɵã�X�Ļ�ѧʽ��Ӧ����N�ĸ���Ϊ����4-4����6=0��Ӧ����H�ĸ���Ϊ��4��3��6=2��Ӧ����O�ĸ���Ϊ����5��2-4����6=1���ʿ��ж�X�Ļ�ѧʽΪ��H
2O��
��2��Һ����Һ�ⷴӦ����������������Ӧ���仯ѧ����ʽΪ��2H
2+O
2
2H
2O��
��3�������������ڸ��������·�Ӧ������������������ѧ����ʽΪ��2Al+Fe
2O
3
2Fe+Al
2O
3��
��4������������ˮ��Ӧ���ɵ����������ƺ���������ѧ����ʽΪ��2CaO
2+2H
2O�T2Ca��OH��
2+O
2����
�ʴ�Ϊ����1��H
2O����2��2H
2+O
2
2H
2O����3��2Al+Fe
2O
3
2Fe+Al
2O
3����4��2CaO
2+2H
2O�T2Ca��OH��
2+O
2����
��������1�����������غ㶨�ɵ��۽��ͣ���ѧ��Ӧǰ��ԭ�ӵ��������Ŀ���䣮��֪�ڻ�ѧ��Ӧ����ʽ�У���Ӧ�����������������ԭ�ӵ��������Ŀ��ͬ���ɴ˿��ƶϻ�ѧ��Ӧ����ʽ�з�Ӧ���������Ļ�ѧʽ��
��2�����������غ㶨�ɣ��ڻ�ѧ��Ӧ�У���Ӧǰ��ԭ�ӵ�����û�иı䣬��Ŀû��������ԭ�ӵ�����Ҳû�иı䣮�ݴ�д��Һ����Һ�ⷴӦ�Ļ�ѧ����ʽ��
��3�����������غ㶨�ɣ��ڻ�ѧ��Ӧ�У���Ӧǰ��ԭ�ӵ�����û�иı䣬��Ŀû��������ԭ�ӵ�����Ҳû�иı䣮�ݴ�д��������������Ӧ�Ļ�ѧ����ʽ��
��4�����������غ㶨�ɣ��ڻ�ѧ��Ӧ�У���Ӧǰ��ԭ�ӵ�����û�иı䣬��Ŀû��������ԭ�ӵ�����Ҳû�иı䣮�ݴ�д������������ˮ��Ӧ�Ļ�ѧ����ʽ��
������������Ҫ����ѧ�����������غ㶨���ƶϻ�ѧʽ�������Լ���ȷ��д��ѧ����ʽ������������ʱҪע�⻯ѧ����ʽ����ƽ����ȷ��ע��Ӧ������

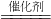 4NO+6X����X�Ļ�ѧʽ��________��
4NO+6X����X�Ļ�ѧʽ��________�� 2H2O��
2H2O�� 2Fe+Al2O3��
2Fe+Al2O3�� 2H2O����3��2Al+Fe2O3
2H2O����3��2Al+Fe2O3 2Fe+Al2O3����4��2CaO2+2H2O�T2Ca��OH��2+O2����
2Fe+Al2O3����4��2CaO2+2H2O�T2Ca��OH��2+O2����


![]() 4NO + 6X ����X�Ļ�ѧʽ�� ��
4NO + 6X ����X�Ļ�ѧʽ�� ��![]() (2)��4�֣� �ҹ�������ƺ�����ij���3�Ż����Һ����Һ�����ƽ��������ʱ������Ӧ�Ļ�ѧ����ʽ ��
(2)��4�֣� �ҹ�������ƺ�����ij���3�Ż����Һ����Һ�����ƽ��������ʱ������Ӧ�Ļ�ѧ����ʽ ��