��2013?������С�Ʒ���ůˮƿ����һ�㵭��ɫ��ˮ������Ϥ���õ�����ˮ���г���̼����⣬���������࣮����̽������û���������ʣ��������ռ�ˮ�����º�ɽ�������ʵ�飺
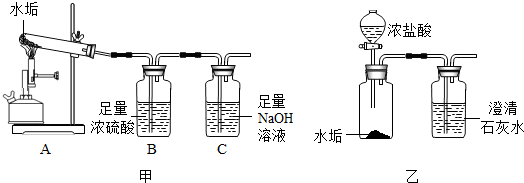
��1��ȡ2.5gˮ����ͼ���е�Aװ�ø��¼��ȳ�ַ�Ӧ����֪CaCO
3CaO+CO
2��������������������ͨ��B��Cװ�ã�ʵ�����Cװ��������������Һ������0.88g��
��д��Cװ���з��ֵĻ�ѧ��Ӧ����ʽ
2NaOH+CO2�TNa2CO3+H2O
2NaOH+CO2�TNa2CO3+H2O
��Bװ���е�Ũ�������������շ�Ӧ�в�����ˮ����������������Ũ�����
��ˮ
��ˮ
�ԣ�
�۸�ˮ������̼��Ƶ���������Ϊ
80%
80%
��
��2����ͼ����ʾ�ķ�����ʵ�飬������ֳ���ʯ��ˮû�б���ǣ�ԭ����
Ũ������лӷ��ԣ����������Ʒ�Ӧ����Ũ�����лӷ��ԣ������ij����������ܽ⣩
Ũ������лӷ��ԣ����������Ʒ�Ӧ����Ũ�����лӷ��ԣ������ij����������ܽ⣩
��




 ���Ͱ�ͨ��ĩ���ϵ�д�
���Ͱ�ͨ��ĩ���ϵ�д�