
 ijͬѧΪ�˼�����ﹺ�������炙����Ƿ�ϸ�����ȡ27.5g������Ʒ������Ũ�ռ���Һ���ȣ������İ�����100.0g���������գ�������հ�������Һ������m�뷴Ӧʱ��t�ı仯����ͼ��ʾ�����漰�ķ�ӦΪ����NH4��2SO4+2NaOH�TNa2SO4+2H2O+2NH3���� 2NH3+H2SO4�T��NH4��2SO4������㣺
ijͬѧΪ�˼�����ﹺ�������炙����Ƿ�ϸ�����ȡ27.5g������Ʒ������Ũ�ռ���Һ���ȣ������İ�����100.0g���������գ�������հ�������Һ������m�뷴Ӧʱ��t�ı仯����ͼ��ʾ�����漰�ķ�ӦΪ����NH4��2SO4+2NaOH�TNa2SO4+2H2O+2NH3���� 2NH3+H2SO4�T��NH4��2SO4������㣺 =5.6g���ʻ����е�Ԫ�ص���������Ϊ
=5.6g���ʻ����е�Ԫ�ص���������Ϊ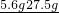 ��100%=20.4%�������ֻ������ںϸ��Ʒ��
��100%=20.4%�������ֻ������ںϸ��Ʒ��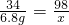
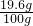 ��100%=19.6%
��100%=19.6%

 ͨ��ѧ��Ĭд����ϵ�д�
ͨ��ѧ��Ĭд����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
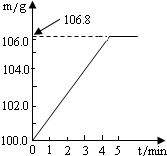
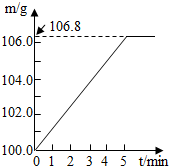 ijͬѧΪ�˼�����ﹺ�������炙����Ƿ�ϸ�ȡ��27.5g������Ʒ������Ũ�ռ���Һһ����ȣ������İ�����������100.0g���������գ�������հ�������Һ������m�뷴Ӧʱ��t�ı仯����ͼ��ʾ����֪�ϸ�����炙����к��������ͣ�20%�����漰�ķ�ӦΪ��NH4��2SO4+2NaOH=Na2SO4+2H2O+2NH3����2NH3+H2SO4=��NH4��2SO4���Լ��㣺
ijͬѧΪ�˼�����ﹺ�������炙����Ƿ�ϸ�ȡ��27.5g������Ʒ������Ũ�ռ���Һһ����ȣ������İ�����������100.0g���������գ�������հ�������Һ������m�뷴Ӧʱ��t�ı仯����ͼ��ʾ����֪�ϸ�����炙����к��������ͣ�20%�����漰�ķ�ӦΪ��NH4��2SO4+2NaOH=Na2SO4+2H2O+2NH3����2NH3+H2SO4=��NH4��2SO4���Լ��㣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��2012?����ض�ģ��ijͬѧΪ�˼�����ﹺ�������炙����Ƿ�ϸ�����ȡ27.5g������Ʒ������Ũ�ռ���Һ���ȣ������İ�����100.0g���������գ�������հ�������Һ������m�뷴Ӧʱ��t�ı仯����ͼ��ʾ�����漰�ķ�ӦΪ����NH4��2SO4+2NaOH�TNa2SO4+2H2O+2NH3���� 2NH3+H2SO4�T��NH4��2SO4������㣺
��2012?����ض�ģ��ijͬѧΪ�˼�����ﹺ�������炙����Ƿ�ϸ�����ȡ27.5g������Ʒ������Ũ�ռ���Һ���ȣ������İ�����100.0g���������գ�������հ�������Һ������m�뷴Ӧʱ��t�ı仯����ͼ��ʾ�����漰�ķ�ӦΪ����NH4��2SO4+2NaOH�TNa2SO4+2H2O+2NH3���� 2NH3+H2SO4�T��NH4��2SO4������㣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��2011?��ƽ����ģ��ijͬѧΪ�˼�����ﹺ�������炙����Ƿ�ϸ�����ȡ27.5g������Ʒ������Ũ�ռ���Һ���ȣ������ĵ�����100.0g���������գ�������հ�������Һ������m�뷴Ӧʱ��t�ı仯��ͼ��ʾ�����漰�ķ�ӦΪ����NH4��SO4+2NaOH=Na2SO4+2NH3+2H2O����2NH3+H2SO4=��NH4��SO4������㣺��1����ȫ��Ӧ���������
��2011?��ƽ����ģ��ijͬѧΪ�˼�����ﹺ�������炙����Ƿ�ϸ�����ȡ27.5g������Ʒ������Ũ�ռ���Һ���ȣ������ĵ�����100.0g���������գ�������հ�������Һ������m�뷴Ӧʱ��t�ı仯��ͼ��ʾ�����漰�ķ�ӦΪ����NH4��SO4+2NaOH=Na2SO4+2NH3+2H2O����2NH3+H2SO4=��NH4��SO4������㣺��1����ȫ��Ӧ����������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ijͬѧΪ�˼�����ﹺ�������炙����Ƿ�ϸ�ȡ��27.5g������Ʒ������Ũ�ռ���Һһ����ȣ������İ�����������100.0g���������գ�������հ�������Һ������m�뷴Ӧʱ��t�ı仯����ͼ��ʾ����֪�ϸ�����炙����к�����������20%�����漰�ķ�ӦΪ��NH4��2SO4+2NaOH=Na2SO4+2H2O+2NH3����2NH3+H2SO4=��NH4��2SO4���Լ��㣺
ijͬѧΪ�˼�����ﹺ�������炙����Ƿ�ϸ�ȡ��27.5g������Ʒ������Ũ�ռ���Һһ����ȣ������İ�����������100.0g���������գ�������հ�������Һ������m�뷴Ӧʱ��t�ı仯����ͼ��ʾ����֪�ϸ�����炙����к�����������20%�����漰�ķ�ӦΪ��NH4��2SO4+2NaOH=Na2SO4+2H2O+2NH3����2NH3+H2SO4=��NH4��2SO4���Լ��㣺�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com