
| ϡ��������� |
��һ�μ���10g |
�ڶ��μ���10g |
�������10g |
���Ĵμ���10g |
| ʣ���������� | 3.0g | 2.0g | 1.0g | 0.4g |
| ̼��Ƶ����� |
| ʯ��ʯ��Ʒ������ |
| 4g-0.4g |
| 4g |
| 100 |
| 1g |
| 73 |
| x |
| 0.73g |
| 10g |
| 100 |
| 3.6g |
| 44 |
| y |


 �������¿��ÿ�ʱ��ҵϵ�д�
�������¿��ÿ�ʱ��ҵϵ�д� Ӣ�żƻ�ͬ����ʱ��Чѵ��ϵ�д�
Ӣ�żƻ�ͬ����ʱ��Чѵ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
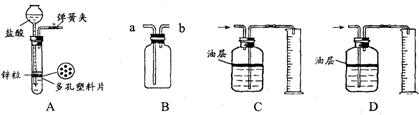
 һ��ѧ��ȤС���ij�±���װ���еġ����������ܺ��棬���ǹ۲쵽�������������װ��ע�ijɷ�Ϊ���ۡ�����̿���Ȼ��ƣ����ֻҺ�ɫ�Ĺ����л���������������ɫ��ĩ��
һ��ѧ��ȤС���ij�±���װ���еġ����������ܺ��棬���ǹ۲쵽�������������װ��ע�ijɷ�Ϊ���ۡ�����̿���Ȼ��ƣ����ֻҺ�ɫ�Ĺ����л���������������ɫ��ĩ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
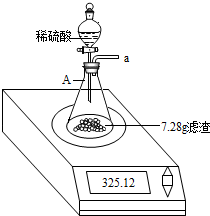
 ��2013?�����ж�ģ����ѧ��ȤС���ijƷ��������̼��ƺ�����������̽����������Ħ������Ҫ��̼��ơ�����������ɣ������ɷ���������ʱ���������ɣ���������ͼ��ʾװ�ã�ͼ�мг�������ȥ������ʵ�飬��ַ�Ӧ�ⶨB�����ɵ�BaCO3������������ȷ��̼��Ƶ�������������ش��������⣮
��2013?�����ж�ģ����ѧ��ȤС���ijƷ��������̼��ƺ�����������̽����������Ħ������Ҫ��̼��ơ�����������ɣ������ɷ���������ʱ���������ɣ���������ͼ��ʾװ�ã�ͼ�мг�������ȥ������ʵ�飬��ַ�Ӧ�ⶨB�����ɵ�BaCO3������������ȷ��̼��Ƶ�������������ش��������⣮�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
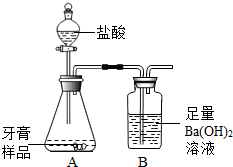
 ��ѧ��ȤС���ijƷ��������̼��ƺ�����������̽����������Ħ������Ҫ��̼��ơ�����������ɣ������ɷ���������ʱ���������ɣ���������ͼ��ʾװ�ã�ͼ�мг�������ȥ������ʵ�飬��ַ�Ӧ�ⶨB�����ɵ�BaCO3������������ȷ��̼��Ƶ�������������ش��������⣮
��ѧ��ȤС���ijƷ��������̼��ƺ�����������̽����������Ħ������Ҫ��̼��ơ�����������ɣ������ɷ���������ʱ���������ɣ���������ͼ��ʾװ�ã�ͼ�мг�������ȥ������ʵ�飬��ַ�Ӧ�ⶨB�����ɵ�BaCO3������������ȷ��̼��Ƶ�������������ش��������⣮�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
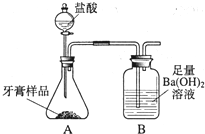
 һ��ѧ��ȤС���ij�±���װ���еġ����������ܺ��棬���ǹ۲쵽�������������װ��ע�ijɷ�Ϊ���ۡ�����̿���Ȼ��ƣ����ֻҺ�ɫ�Ĺ����л���������������ɫ��ĩ��
һ��ѧ��ȤС���ij�±���װ���еġ����������ܺ��棬���ǹ۲쵽�������������װ��ע�ijɷ�Ϊ���ۡ�����̿���Ȼ��ƣ����ֻҺ�ɫ�Ĺ����л���������������ɫ��ĩ���鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com