ijͬѧȡһ���������ڿ����еĹ����ռ���Ʒ������ֻ����NaOH��Na2CO3����������ˮ�У������м���������ϡH2SO4��Һ����ֽ��赽���ٲ�������Ϊֹ����÷ų�CO2���������Ϊ2.2g����ش�
��1��д��������Ʒ��������ϡH2SO4��Һ������Ӧ�Ļ�ѧ����ʽ��______����______��
��2����������ȡ��Ʒ������Ϊ20g��������Ʒ��NaOH������������д����ϸ�ļ�����̣�
��3����ȡ������ȵĹ����ռ����ݣ����ܷ�����ҷ�¶�ÿ���һ��ʱ��ʹ�䲿�ֱ��ʣ�Ȼ�����Ƿֱ�����ˮ��������ͬŨ�ȵ�ϡ�����ַ�Ӧ��������ϡ������Һ������Ƚϣ�V���ף�______V���ң����=����������������
���𰸡�
��������1���������������������Һ��Ӧ���������ƺ�ˮ�Լ�̼������Һ�����ᷴӦ���������ơ�ˮ�Ͷ�����̼���н��
��2�����������غ㶨�ɣ����ö�����̼�������������Ʒ��NaOH������������������Ʒ��NaOH������������
��3�����������غ㶨�ɣ���������ת����̼���ƵĹ����У���Ԫ�ص�����û�䣬����Ԫ�������غ�ĽǶȿ��Խ��ʹ��⣮
����⣺��1�����������������Һ��Ӧ���������ƺ�ˮ����Ӧ�Ļ�ѧ����ʽΪ2NaOH+H
2SO
4�TNa
2SO
4+2H
2O��̼������Һ�����ᷴӦ���������ơ�ˮ�Ͷ�����̼����Ӧ�Ļ�ѧ����ʽΪNa
2CO
3+H
2SO
4�TNa
2SO
4+H
2O+CO
2�������2NaOH+H
2SO
4�TNa
2SO
4+2H
2O��Na
2CO
3+H
2SO
4�TNa
2SO
4+H
2O+CO
2����
��2�����ռ���Ʒ��Na
2CO
3������Ϊx��
Na
2CO
3+H
2SO
4�TNa
2SO
4+H
2O+CO
2��
106 44
x 2.2g
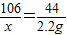
x=5.3 g
��Ʒ��NaOH����������Ϊ��
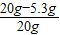
×100%=73.5%
����Ʒ��NaOH����������73.5%��
��3�����������غ㶨�ɣ���������ת����̼���ƵĹ����У���Ԫ�ص�����û�䣬���������ƺ�̼���ƶ���ÿ46g��Ԫ������142g�����ƣ�����98g���ᣬ��������ϡ������Һ�������ȣ����=��
������������Ҫ���ڼ�Ļ�ѧ���ʵĻ����Ͽ����й������غ㶨�ɵ��й�֪ʶ���Լ�����������������ļ������⣬ע��ѧ���ۺ����������������п��Ŀ���֮һ��

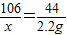
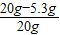 ×100%=73.5%
×100%=73.5%

