

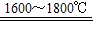 Si+2CO��
Si+2CO��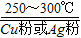 SiHCl3+H2��
SiHCl3+H2��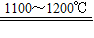 Si+3HCl
Si+3HCl


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��2011���Ĵ���ɽ��36�⣩�ݱ�����̫���ܹ���������Ϻ��������ϵõ��˴��
ģ��Ӧ�ã���װ����������4.68���ߡ�
��1����չ̫���ܲ�ҵ��Ҫ�ŵ��ǣ���һ�㼴�ɣ� ��
��2��̫���ܹ��������ؼ��IJ����Ǹߴ��裬��ͼ������ij�ྦྷ��������ҵ�����ߴ��������ʾ��ͼ��
�����ķ�ӦΪ��
��SiO2+2C Si+2CO��
��Si+3HCl SiHCl3+H2��
�� SiHCl3+H2 Si+3HCl
�����Ʊ����̱���ﵽ��ˮ��������H2��ԭSiHCl3������������O2����������ĺ���� ��
��3��Ϊ�˴ﵽ��ɫ��ѧ�ͽ�Լ��Դ��Ŀ�ģ�����������ij��������Ҫѭ��ʹ�ã������ʵĻ�ѧʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

 Si+2CO��
Si+2CO�� SiHCl3+H2��
SiHCl3+H2�� Si+3HCl
Si+3HCl�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011��ȫ���п���ѧ����������15 ȼ�ϡ�ʹ��ȼ�϶Ի�����Ӱ�� ���ͣ��ƶ���
��2011���Ĵ���ɽ��36�⣩�ݱ�����̫���ܹ���������Ϻ��������ϵõ��˴��
ģ��Ӧ�ã���װ����������4.68���ߡ�
��1����չ̫���ܲ�ҵ��Ҫ�ŵ��ǣ���һ�㼴�ɣ� ��
��2��̫���ܹ��������ؼ��IJ����Ǹߴ��裬��ͼ������ij�ྦྷ��������ҵ�����ߴ��������ʾ��ͼ��
�����ķ�ӦΪ��
��SiO2+2C  Si+2CO��
Si+2CO��
��Si+3HCl  SiHCl3+H2��
SiHCl3+H2��
�� SiHCl3+H2 Si+3HCl
Si+3HCl
�����Ʊ����̱���ﵽ��ˮ��������H2��ԭSiHCl3������������O2����������ĺ���� ��
��3��Ϊ�˴ﵽ��ɫ��ѧ�ͽ�Լ��Դ��Ŀ�ģ�����������ij��������Ҫѭ��ʹ�ã������ʵĻ�ѧʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011��ȫ���п���ѧ����������15ȼ�ϡ�ʹ��ȼ�϶Ի�����Ӱ�� ���ͣ���Ϣ������
��2011���Ĵ���ɽ��36�⣩�ݱ�����̫���ܹ���������Ϻ��������ϵõ��˴��
ģ��Ӧ�ã���װ����������4.68���ߡ�
��1����չ̫���ܲ�ҵ��Ҫ�ŵ��ǣ���һ�㼴�ɣ� ��
��2��̫���ܹ��������ؼ��IJ����Ǹߴ��裬��ͼ������ij�ྦྷ��������ҵ�����ߴ��������ʾ��ͼ��

�����ķ�ӦΪ��
��SiO2+2C  Si+2CO��
Si+2CO��
��Si+3HCl  SiHCl3+H2��
SiHCl3+H2��
�� SiHCl3+H2  Si+3HCl
Si+3HCl
�����Ʊ����̱���ﵽ��ˮ��������H2��ԭSiHCl3������������O2����������ĺ���� ��
��3��Ϊ�˴ﵽ��ɫ��ѧ�ͽ�Լ��Դ��Ŀ�ģ�����������ij��������Ҫѭ��ʹ�ã������ʵĻ�ѧʽ�� ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com