
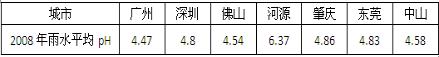
2008����ʡ���ֵ���������ˮƽ��pH���±�����ش��������⣺
| ���� | ���� | ���� | ��ɽ | ��Դ | ���� | ��ݸ | ��ɽ |
| 2008����ˮƽ��pH | 4.47 | 4.8 | 4.54 | 6.37 | 4.86 | 4.83 | 4.58 |
��1���ϱ��оٵ�7�������У�û��������Ⱦ�ĵ�����_______�������������Ҫ������_________�������)�� ��CO ��CO2 ��SO2 ��NO2 ��
��2�����������Ҫ�ɷ�ΪH2SO4��HNO3����д�����л�ѧ����ʽ��
(a)��H2SO4�����긯ʴ�����ҵ�ʯ��ʯ ��
(b)��HNO3��������������������ʯ�ҷ�Ӧ ��
��3��Ϊ�˸������컷��������ӭ������ġ���ɫ���˻ᡱ�����д�ʩ���������������__________�������)�����ƹ������Դ �ڼ�����úֱ����ȼ�� ����̭β������������ ������ȼ���̻����� �����Ʋ��г�ʹ�����ϴ���
 �߽�������ϵ�д�
�߽�������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ���� | ���� | ���� | ��ɽ | ��Դ | ���� | ÷�� | ��ɽ |
| 2008����ˮƽ��pH | 4.47 | 4.8 | 4.54 | 6.37 | 4.86 | 4.83 | 4.58 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013��㶫ʡ�����и�Ȫ����ƥ��ѧУ���꼶3�·�������⻯ѧ�Ծ����������� ���ͣ��ʴ���
��7�֣�2008����ʡ���ֵ���������ˮƽ��pH���±�����ش��������⣺
| ���� | ���� | ���� | ��ɽ | ��Դ | ���� | ��ݸ | ��ɽ |
| 2008����ˮƽ��pH | 4.47 | 4.8 | 4.54 | 6.37 | 4.86 | 4.83 | 4.58 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012��㶫ʡտ��һ�н��廪��ѧУ���꼶���Ĵδ�ѧ�Ծ��������棩 ���ͣ������
| ���� | ���� | ���� | ��ɽ | ��Դ | ���� | ��ݸ | ��ɽ |
| 2008����ˮƽ��pH | 4.47 | 4.8 | 4.54 | 6.37 | 4.86 | 4.83 | 4.58 |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com