
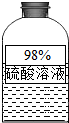
 ��1����ͼ��Ϣ��ƿ����Һ���ڷ��ú�Ũ�ȱ�ϡ��������Ϊ�����ʾ���______�ԣ�������Ͳ��ȡ50ml�ܶ�Ϊ��g/ml��������Һ������Ͳ����Һ����������Ϊ______��ֻҪ����ʽ����
��1����ͼ��Ϣ��ƿ����Һ���ڷ��ú�Ũ�ȱ�ϡ��������Ϊ�����ʾ���______�ԣ�������Ͳ��ȡ50ml�ܶ�Ϊ��g/ml��������Һ������Ͳ����Һ����������Ϊ______��ֻҪ����ʽ����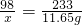
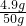 ��100%=9.8%
��100%=9.8%

 ������ʱͬ����ϰ��ϵ�д�
������ʱͬ����ϰ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
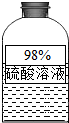 ��2013?������һģ����1����ͼ��Ϣ��ƿ����Һ���ڷ��ú�Ũ�ȱ�ϡ��������Ϊ�����ʾ���
��2013?������һģ����1����ͼ��Ϣ��ƿ����Һ���ڷ��ú�Ũ�ȱ�ϡ��������Ϊ�����ʾ����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�꽭��ʡ��ͨ���������п�һģ��ѧ�Ծ��������棩 ���ͣ������
��1����ͼ��Ϣ��ƿ����Һ���ڷ��ú�Ũ�ȱ�ϡ��������Ϊ�����ʾ����� ���ԣ�������Ͳ��ȡ50ml�ܶ�Ϊ��g/ml��������Һ������Ͳ����Һ����������Ϊ�� ����ֻҪ����ʽ����
��2��ij̽��С��ͬѧ��ij��ҵ��ˮ������H2SO4��HNO3����H2SO4�ĺ������вⶨ��ȡ50g��ˮ���ձ��У���������BaCl2��Һ�����ˡ�ϴ�ӡ������BaSO4����11.65g����ش�
��50g��ˮ��H2SO4������������
��������KOH��Һ���ⶨ50g��ˮ��H2SO4�ĺ�����������ܻ��� ����ѡ�ƫ�͡�����ƫ�ߡ����䡱����ԭ���ǣ��� ����

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�꽭��ʡ��ͨ���������п���ѧһģ�Ծ��������棩 ���ͣ������
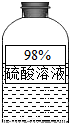
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com