
| ||
| ��Ӧǰ | ��Ӧ�� |
| E������Ϊ100.0g | E������Ϊ102.25g |
| F������Ϊ50.0g | F������Ϊ51.1g |
| ||
| 168 |
| x |
| 44 |
| 1.1g |
| 44 |
| 1.1g |
| 18 |
| y |
| 106a |
| 10.6 |
| 84b |
| 4.2 |
| 18c |
| 1.8 |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
| ��Ӧǰ | ��Ӧ�� |
| E������Ϊ100.0g | E������Ϊ102.25g |
| F������Ϊ50.0g | F������Ϊ51.1g |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| ||
| ��Ӧǰ | ��Ӧ�� |
| E������Ϊ100.0g | E������Ϊ102.25g |
| F������Ϊ50.0g | F������Ϊ51.1g |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
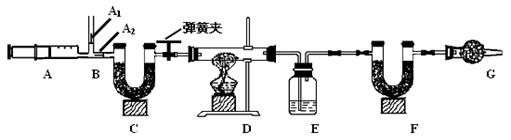
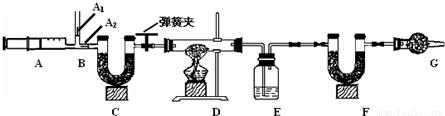
�ҹ��ຣ���ɼ�����Ȼ����Ʒ�ɱ�ʾΪaNa2CO3·bNaHCO3·cH2O��a��b��cΪ��������ȣ���С��ͬѧΪ�ⶨ����ɣ���ȡ����Ȼ����Ʒ16.6g��������ʵ�飺
��֪��1.̼���ƱȽ��ȶ�������ʱ���ֽ⣻2. 2NaHCO3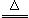 Na2CO3 +CO2 �� +H2O
Na2CO3 +CO2 �� +H2O
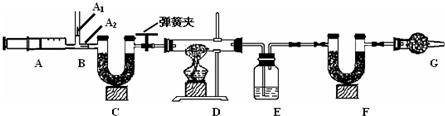
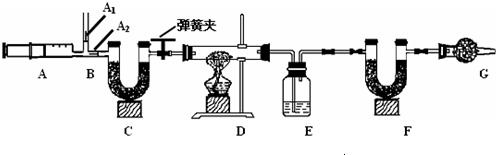
3.ͼ��B��Ϊ����������ע����ʱA1�رգ�A2������ע����ʱ��A1��
������A2�رա�
��һ��ʵ�鲽��
��1��ʵ��ʱ����װ��װ�ã�ʵ��ǰӦ�� �� ��Ȼ��ʵ�鲽���ǣ�
�� ��������ע���� �� ����E��F������ �� �رյ��ɼУ�����D���Թ�ֱ����
Ӧ���ٽ��� �� ���ɼУ��ٴη�����������ע���� �� �ٴγ���E��F��������
����������̽����
��2�� E�е�ҩƷΪ �� ��E�������� �� ��
C��F��G��װ�м�ʯ�ң���ʯ�����ռ�Ĺ����������C�������� �� ��
F�������� �� ��
д�� Fװ������������Ӧ��һ����ѧ����ʽ �� ��
��3��ʵ�鲽�������ܷ�ߵ� �� ����ܡ����ܡ������������в���ܵIJ�����
������õ�̼���������� �� ���ƫ����ƫС��������Ӱ�족����
�ò�������ע����ʱ������Ŀ���� �� ��
��û��Gװ�ã���̼�����Ƶ����� �� ���ƫ����ƫС��������Ӱ�족����
��4���±���ʵ���¼���ݣ�
| ��Ӧǰ | ��Ӧ�� |
| E������Ϊ100.0g | E������Ϊ102.25g |
| F������Ϊ50.0g | F������Ϊ51.1g |
�� �� ̼�����Ʒֽ����ɶ�����̼������Ϊ �� g
�� ̼�����Ƶ�����Ϊ �� g
�� ����Ȼ��Ļ�ѧʽ��a:b:c= �� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2007�꽭��ʡ���о��ݸ���ѧ��ǰ�������Ի�ѧģ���Ծ���4�·ݣ��������棩 ���ͣ������
 Na2CO3��+CO2��+H2O
Na2CO3��+CO2��+H2O| ��Ӧǰ | ��Ӧ�� |
| E������Ϊ100.0g | E������Ϊ102.25g |
| F������Ϊ50.0g | F������Ϊ51.1g |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com