

���� ����Ŀ����Ϣ��֪�������ƹ��̸��ε���Һ�����ڱ�����Һ���Тڢۣ���Ϊ100g20%�� KNO3��Һ�ܽ�20gKNO3 ����ǡ�ôﵽ����״̬���ܺ��ձ�����Һ��������������=$\frac{40g+100g��20%��3}{300g+40g}��100%$��29.4%���ڲ�����ˮ������������£������ƹ����У��²����лᵼ�¢ܺ��ձ�����Һ��������������ƫ����У�����KNO3 ��Һʱ�в�����Һ�������������������ƽ��ȡ40�� KNO3���壮
��� �⣺��1�������ƹ��̸��ε���Һ�����ڱ�����Һ���Тڢۣ���Ϊ100g20%�� KNO3��Һ�ܽ�20gKNO3 ����ǡ�ôﵽ����״̬���ʴ�Ϊ���ڢۣ�
��2���ܺ��ձ�����300g20%���������Һ��40g���壬�����Һ��������������=$\frac{40g+100g��20%��3}{300g+40g}��100%$��29.4%���ʴ�Ϊ��29.4%��
��3���ڲ�����ˮ������������£������ƹ����У��²����лᵼ�¢ܺ��ձ�����Һ��������������ƫ����У�����KNO3 ��Һʱ�в�����Һ��������Ϊˮ���ˣ��������������ƽ��ȡ40�� KNO3���壬��Ϊ���ʳƶ��ˣ��ʴ�Ϊ��BC��
���� ���⿼�����ж��ж��Ƿ��DZ�����Һ�벻������Һ�ķ��������������йصļ����������Һ�����е�ʧ����ɵĺ�����йصļ���Ҫȷ����������Ҫ������ѡ�����������У�


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��Һ����Ϊ��ɫ | |
| B�� | �������������ܵ���ԭп�ۺ�þ�۵����� | |
| C�� | ��Һ�����ٺ������ֽ������� | |
| D�� | ������һ����Ag��Cu |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
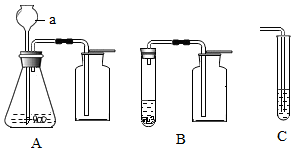 �������ʵ��װ�ã��ش����⣺
�������ʵ��װ�ã��ش����⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
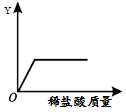 ��ͼ��ʾ����ʢ��һ����ʯ��ʯ��Ʒ�����ʲ�����ˮ�������뷴Ӧ�����ձ��в��ϼ�
��ͼ��ʾ����ʢ��һ����ʯ��ʯ��Ʒ�����ʲ�����ˮ�������뷴Ӧ�����ձ��в��ϼ�| A�� | ˮ������ | B�� | ������̼������ | ||
| C�� | ��Ʒ��̼��Ƶ����� | D�� | ��Һ���Ȼ��Ƶ��������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Na2O+H2O=2NaOH | B�� | 2HCl+Mg��OH��2=MgCl2+2H2O | ||
| C�� | KCl+AgNO3=AgCl��+KNO3 | D�� | BaCl2+H2SO4=2HCl+BaSO4�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
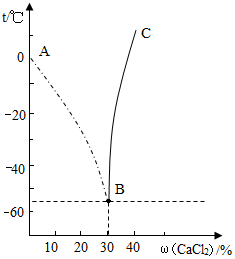 ��һ��������ˮʱ����ʹˮ�ı��㣨���̵㣩���ͣ����㽵�Ͷ�����������Һ�е����������йأ������������Һ���£������������ij�¶Ȼ�������������������Һ�е����������Ƚϴ�ʱ���ڽ���Һ��ȴ�Ĺ������������Ĺ��岻�DZ������εľ��壮��ͼ��ʾ��������Ϊ�Ȼ�����Һ�����ʵ�����������������Ϊ�¶ȣ�
��һ��������ˮʱ����ʹˮ�ı��㣨���̵㣩���ͣ����㽵�Ͷ�����������Һ�е����������йأ������������Һ���£������������ij�¶Ȼ�������������������Һ�е����������Ƚϴ�ʱ���ڽ���Һ��ȴ�Ĺ������������Ĺ��岻�DZ������εľ��壮��ͼ��ʾ��������Ϊ�Ȼ�����Һ�����ʵ�����������������Ϊ�¶ȣ��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
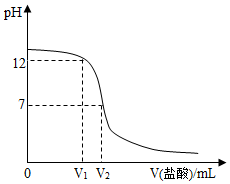 ijʵ��С�����кͷ�Ӧ��ʵ�飬��ʢ������������Һ�����з�̪��Һ�����ձ�����μ���ϡ���ᣬ�����Ͻ��裮��ͼΪ����ʵ�����ݻ��Ƶ� V�����ᣩ-pHͼ��
ijʵ��С�����кͷ�Ӧ��ʵ�飬��ʢ������������Һ�����з�̪��Һ�����ձ�����μ���ϡ���ᣬ�����Ͻ��裮��ͼΪ����ʵ�����ݻ��Ƶ� V�����ᣩ-pHͼ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ѡ�� | ���� | �������� | ��ȥ���ʵķ��� |
| A | NaCl��Һ | Na2CO3 | ��������ϡ����������ᾧ |
| B | KCl | MnO2 | ��������ˮ�ܽ⡢���ˡ������ᾧ |
| C | ϡ���� | ϡ���� | ���������Ȼ�����Һ������ |
| D | CaO | CaCO3 | ����������ˮ�ܽ⡢���ˡ������ᾧ |
| A�� | A�� | B�� | B�� | C�� | C�� | D�� | D�� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com