
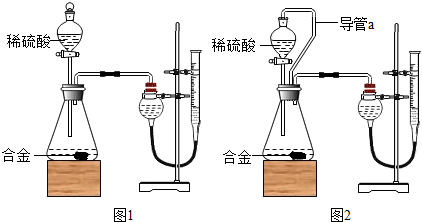
| 65 |
| X |
| 2 |
| 0.089g |
| 2.89g |
| 10g |
| 65 |
| Y |
| 2 |
| 0.2g |
| 6.5g |
| 38% |



| Дкј¶ | ёЯЦРїОіМ | Дкј¶ | іхЦРїОіМ |
| ёЯТ» | ёЯТ»Гв·СїОіМНЖјцЈЎ | іхТ» | іхТ»Гв·СїОіМНЖјцЈЎ |
| ёЯ¶ю | ёЯ¶юГв·СїОіМНЖјцЈЎ | іх¶ю | іх¶юГв·СїОіМНЖјцЈЎ |
| ёЯИэ | ёЯИэГв·СїОіМНЖјцЈЎ | іхИэ | іхИэГв·СїОіМНЖјцЈЎ |
їЖДїЈєіхЦР»ЇС§ АґФґЈє МвРНЈє

Ійїґґр°ёєНЅвОц>>
їЖДїЈєіхЦР»ЇС§ АґФґЈє МвРНЈєЅвґрМв

Ійїґґр°ёєНЅвОц>>
їЖДїЈєіхЦР»ЇС§ АґФґЈє МвРНЈє
ЅрКфІДБПФЪЙъІъЎўЙъ»оЦРУРЧЕ№г·єµДУ¦УГЎЈ
 ўЕПВБРЙъ»оУГЖ·Ј¬ЦчТЄАыУГЅрКфѕЯУРБјєГµјИИРФµДКЗЈє___ ____ЎЈ
ўЕПВБРЙъ»оУГЖ·Ј¬ЦчТЄАыУГЅрКфѕЯУРБјєГµјИИРФµДКЗЈє___ ____ЎЈ
AЈ®ЦэМъґ¶ѕЯ BЈ®ЅрКфПёЛї CЈ®НЦЖµзАВ D.Мъґё
ўЖ№¤ТµЙъІъЦРЈ¬ЗРёоМъ°еК±УГБтЛбНИЬТєФЪМъ°еЙП»ПЯїЙБфПВємЙ«µДУЎјЈЎЈУР№Ш·ґУ¦µД»ЇС§·ЅіМКЅОЄ____ _ _ЎЈ
ўЗИфТЄ±ИЅПМъЎўГМЎўНµДЅрКф»о¶ЇРФЗїИхЈ¬їЙСЎФсµДТ©Ж·іэБЛМъЎўНµҐЦКНвЈ¬»№РиТЄ ЎЈ
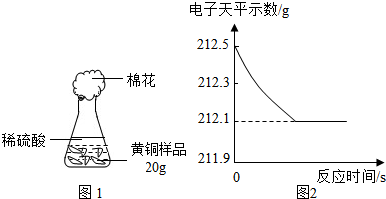
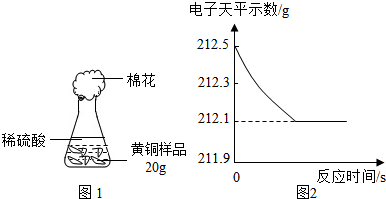
ўИСРѕїРФС§П°РЎЧйОЄБЛІв¶Ё»ЖНЈЁНЎўРїєПЅрЈ©µДЧйіЙЈ¬УГµзЧУМмЖЅ·Ц±ріЖµГЧ¶РОЖїУлГЮ»ЁµДЦКБїОЄ44.1gЈ¬іЖИЎ»ЖН
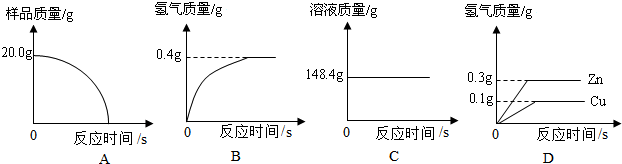
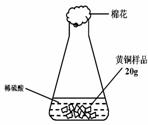
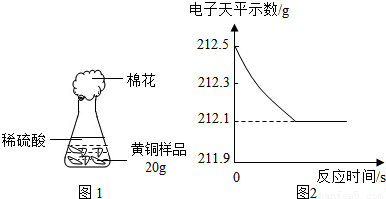
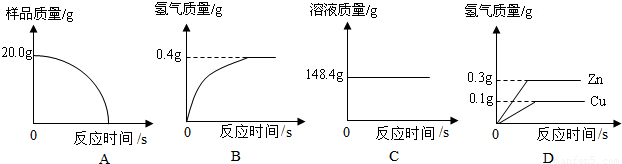
СщЖ·20.0gЎЈФЪЧ¶РОЖїЦРјУИлёГСщЖ·єНЧгБїПЎБтЛбєуЖїїЪИыЙПГЮ»ЁЈ¬ИзНј1ЛщКѕЎЈЅ«µзЧУМмЖЅіЖБїµДКэѕЭ»жіЙНј2ЎЈ
ЗлИПХж·ЦОцКэѕЭЈ¬»ШґрПВБРОКМвЈє
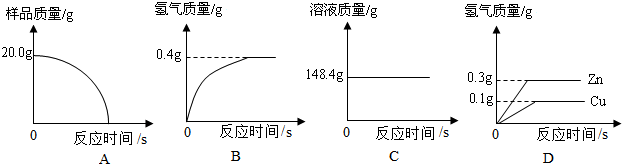
ЈЁўсЈ©ЛДёцН¬С§ґУ¶аЅЗ¶Иґ¦АнКэѕЭЈ¬ПВБРКэѕЭґ¦АнµДНјПсЦРХэИ·µДКЗ ЎЈ
ЈЁўтЈ©КФјЖЛгЈє
ўЩСщЖ·ЦРНµДЦКБї·ЦКэЈ»ўЪЗЎєГ·ґУ¦К±ЛщµГИЬТєЦРИЬЦКµДЦКБї·ЦКэЎЈ

Ійїґґр°ёєНЅвОц>>
їЖДїЈєіхЦР»ЇС§ АґФґЈє2011ДкЅЛХКЎДПѕ©КРЅЁЪюЗшЦРїј»ЇС§Т»ДЈКФѕнЈЁЅвОц°жЈ© МвРНЈєЅвґрМв

Ійїґґр°ёєНЅвОц>>
№ъјКѧУУЕСЎ - Б·П°ІбБР±н - КФМвБР±н
єю±±КЎ»ҐБЄНшОҐ·ЁєНІ»БјРЕПўѕЩ±ЁЖЅМЁ | НшЙПУРє¦РЕПўѕЩ±ЁЧЁЗш | µзРЕХ©ЖѕЩ±ЁЧЁЗш | ЙжАъК·РйОЮЦчТеУРє¦РЕПўѕЩ±ЁЧЁЗш | ЙжЖуЗЦИЁѕЩ±ЁЧЁЗш
ОҐ·ЁєНІ»БјРЕПўѕЩ±Ёµз»°Јє027-86699610 ѕЩ±ЁУКПдЈє58377363@163.com