
 ��2002?�½����ס�����λͬѧ����ͼ��ʾ��װ����ȡ���壬��װ���ɴ�С�����Թ�������ɣ�����С�Թ�Ϊ���巢���������Թ�Ϊ�����ռ�װ�ã���ͬѧ�ô�װ����ȡH2����ͬѧ�ô�װ����ȡCO2������Ӧ��Ϻ������Թ����ϳ��������Ĵָ��ס�Թܿڣ���ش�
��2002?�½����ס�����λͬѧ����ͼ��ʾ��װ����ȡ���壬��װ���ɴ�С�����Թ�������ɣ�����С�Թ�Ϊ���巢���������Թ�Ϊ�����ռ�װ�ã���ͬѧ�ô�װ����ȡH2����ͬѧ�ô�װ����ȡCO2������Ӧ��Ϻ������Թ����ϳ��������Ĵָ��ס�Թܿڣ���ش�

 һ����ʦ�����Ծ�ϵ�д�
һ����ʦ�����Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
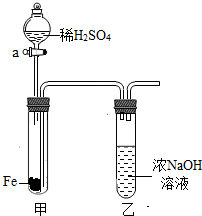 ��2002?�½�����ͼ��ʾ�����Թ������ȷ���һ��������Ƭ��©����ʢ��ϡH2SO4�����Թ���ʢ��Ũ��Һ��
��2002?�½�����ͼ��ʾ�����Թ������ȷ���һ��������Ƭ��©����ʢ��ϡH2SO4�����Թ���ʢ��Ũ��Һ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com