
����Ŀ������ͼ��ʾװ�ý���ʵ�飬�о�ȼ�յ�������
��֪�������Ż��Ϊ40�棬�����Ż��Ϊ240�档
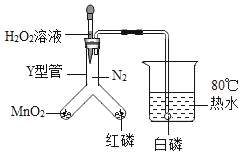
��1����H2O2��Һ��MnO2�Ӵ�ʱ��������Ӧ�Ļ�ѧ ����ʽΪ_____���÷�Ӧ����_____��Ӧ�����������Ӧ���ͣ�
��2����Y���м���H2O2��Һ�۲쵽���ܿڿ�ʼ��������ʱ���ձ��а��ײ�ȼ�գ�һ��ʱ�����ȼ�գ��������ܹ�֤���Ŀ�ȼ��ȼ�յ�������_____����֤����ȼ��ȼ�յ���һ�����������ݵ�������_____��
���𰸡�2H2O2![]() 2H2O+O2�� �ֽ� ��Ҫ�������Ӵ� �ձ��а���ȼ��ʱ��Y���к��ײ�ȼ��
2H2O+O2�� �ֽ� ��Ҫ�������Ӵ� �ձ��а���ȼ��ʱ��Y���к��ײ�ȼ��
��������
��1�����������ڶ������̵Ĵ�����������ˮ����������Ӧ�Ļ�ѧ����ʽΪ��2H2O2![]() 2H2O+O2�����÷�Ӧ������һ���������ʽ�����ϷֽⷴӦ�����������ڷֽⷴӦ��
2H2O+O2�����÷�Ӧ������һ���������ʽ�����ϷֽⷴӦ�����������ڷֽⷴӦ��
��2����Y���м���H2O2��Һ�۲쵽���ܿڿ�ʼ��������ʱ���ձ��а��ײ�ȼ�գ�һ��ʱ�����ȼ�գ�֤���Ŀ�ȼ��ȼ�յ���������Ҫ�������Ӵ����ձ��а���ȼ��ʱ��Y���к��ײ�ȼ�գ�˵����ȼ��ȼ�յ��������¶�Ҫ�ﵽ��ȼ����Ż�㡣



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʵ�����з���һƿ���ڷ��ö������������ۡ�ȡ6.0��������Ʒ����100��ϡ������μ������У��������������������Һ�����ı仯�����ͼ��ʾ��ʵ������в�ò�������0.1�ˡ�������Һ��������������Ϊ��������
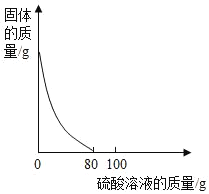
A. 24.5%B. 40.2%C. 47.3%D. 60.9%
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij������ȤС���ͬѧ�Զ�����̼����ȡ�����ʽ������̽����
��ʵ��عˣ�
(1)ʵ�����ô���ʯ��ϡ������ȡ������̼�Ļ�ѧ����ʽΪ______���������ſ������ռ�������̼��ԭ����______��
��ʵ��̽����
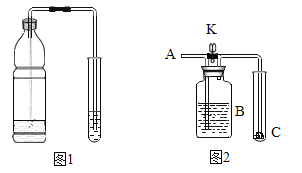
(2)��ͼ1��ʾ������ˮƿ�ǣ��д�������ð�������ϴ����ܵ���Ƥ����������һ������װ�еμ�����ɫʯ��Һ������ˮ���Թ��У���������ˮƿ���۲쵽��ɫʯ����Һ���;���������Թܣ�������Һ��ɫ�ɺ�ɫ��Ϊ��ɫ��д�������仯���з�Ӧ�Ļ�ѧ����ʽ______��______��
(3)����ˮƿ��ʱ���д�������ð����˵��������ܽ��______��
(4)��ͼ2��ʾ����C��������ʯ����ҺȾ����ɫ�ĸ����ֽ���������ʵ�飺
��A��������������ͨ������X�����ر�Kʱ��C������ɫʯ��ֽ������ɫ;����K��C������ɫʯ��ֽ����Ϊ��ɫ��������XΪδ������Ķ�����̼����Bƿ��ʢ�ŵ���Һ����Ϊ______(����ĸ)��������XΪδ�������һ����Ȼ���Ķ�����̼����Bƿ��ʢ�ŵ���Һ����Ϊ_____(����ĸ)
A Ũ���� B ����������Һ C ����̼��������Һ
��������˼��
(5)��С���ÿ�����Ʒ����߶�����̼�����Ŀ�������Ʒ��������ͬ�����ⶨ�����ڹ���ǰ���¶ȵı仯��ʵ������ͼ3�����������Ʒ��Ӧ���¶ȱ仯������__________(����a������b��)��������̼���Բ�������ЧӦ��

���������ЧӦ���������滷���Ĺ����______(��дһ��)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʵ��������ʯ��ʯ��ϡ���ᡢ�{����ء����ľ�顢ҩ�ס����Ӽ�������������ش��������⼰��д�հס�
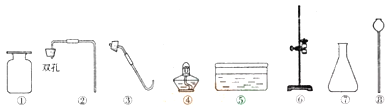
(1)������һ��������_________(�����ƣ�������������������������ȡһ�����壬������Ӧ�Ļ�ѧ����ʽΪ��________________.������Ţ������ڴ�ʵ���е������Ǣ�________________��
(2)�ô��е��ܵ����������Թ�,���װ�õ������ԡ�ȷ��װ�ò�©���ο����������Թ���װ�������ĸ�����ط�ĩ�������Թܿڢ�__________________�ô��е��ܵ����������ܿڣ�֮��IJ����Ǣ�__________________________________________��
(3)�뽫��ͼ��ȡ���������е�װ��ͼ����������____________;

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ѧ�ڴٽ���ᷢչ��������������������淢���Ų�����������á�
��1���й��й���Խ��ӵ�ϰ�ס��������ӵ�ԭ����С��ۡ����ڡ��߲ˡ�ֲ���͡���ζ���ȡ�
��С����и����Ļ���Ӫ������______��
�������еĵ��������ɶ���______������ĸ�����ɵļ�Ϊ���ӵĻ����
a������ b������ c��������
����ǿ�������dz��õĵ�ζ��������ʳ�ÿ���Ԥ��______������ĸ����
a���������� b��ƶѪ c����״���״�
��2��������������������Ӧ�ù㷺��
��������Ʒ�У����л��ϳɲ����Ƴɵ���______������ĸ����
a��![]() ������Ͱ b��
������Ͱ b��![]() ����ñ�� c��
����ñ�� c��![]() ̼����
̼����
��C919�ɻ�������Ƥʹ������﮺Ͻ���ϡ����в�������﮺Ͻ����ʵ���_____������ĸ����

a���ܶȴ� b��Ӳ�ȸ� c������ʴ
��3���й��϶��ĺ�����̽�����״�֤ʵ������ᣵ���Ҫ����֮һ��Fe2SiO4����֪Fe2SiO4�й�Ԫ�صĻ��ϼ�Ϊ��4����������Ԫ�صĻ��ϼ�Ϊ_____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ͼ������ȷ��ӳ��Ӧ�仯��ϵ����
|
|
|
|
A����һ��������AgNO3��Cu(NO3)2�Ļ����Һ�в��ϼ������� | B����Ũ����¶���ڿ����� | C. �ں��������£������͵�NaCl��Һ��������ˮ | D����һ�������ı���ʯ��ˮ�м�����ʯ�� |
A. A B. B C. C D. D
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����4cm����þ������һ������ϡHC1�У������������ݺ���ְ�ɫ���塣С��ͬѧΪ̽����ɫ������ijɷֽ�������ʵ�顣
�����ϣ���MgCl2��Һ�У������·�����Ӧ��Mg + 2H2O = Mg(OH)2��+ H2����
�����룩
�����ɵ�MgCl2��������� ��Ӧ����þ��ʣ�� ��������Mg(OH)2����
��ʵ�飩��ͬ3��ʵ�飬���˲�������ˮϴ�ӹ��壬ֱ�����һ��ϴ��������Һ�еμ���������Һ��___���������ֱ�õ�����ྻ����m g��
��1���Ա�ʵ�飺��m g������m g____���ֱ����5 mL����ˮ�У�������۲�����������Լ��١�
���ۣ����������
��2��ȡm g���壬����ϡ���ᡣ���۲쵽___����
���ۣ������Ҳ��������
��3��ȡm g���壬ȫ������ϡ���ᣬ��������Һ�ֳ����ȷݡ�
����һ����Һ�еμӹ�����NaOH��Һ��������1.16gMg(OH)2����;
������һ����Һ�еμӹ�����AgNO3��Һ��������2.87 g������
���ۣ������Ҳ������������֪��ɫ���廯ѧʽΪ[ Mgx(OH)y z]����ȱ�ٵ�Ԫ�ط���Ϊ___��
�����ۣ����������x:z= ____��x:y= ____��
��д���õ���ɫ�����H2�Ļ�ѧ����ʽ����֪����������Ϊ1��2����___��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ڲ�����ļ�������������Ϊ�ֳֵ���Ҷ�ݵ�������������è�����������Ҹ�����ѧ��������Ҳӭ����һֻ�ֳ���Ҷ�ݵ�������������X����ͼ��ʾ����Ҷ�ݵ�Ҷ����A��B��C��D�������ʹ��ɣ���������ҶƬ֮��ᷢ����Ӧ������C��D�Ǻ�ɫ���壬B��CΪ���ʣ�A��DΪ�����
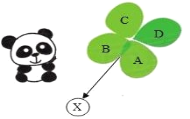
��1������CΪ_____������B�������е�һ����;��_____��
��2��A��D��Ӧ�Ļ�ѧ����ʽ������_____��_____��
��3���ֳ���Ҷ�ݵ�������������X�����㣺A��B��C��D�ֱ�ͨ��һ����Ӧ��ֱ��ת��ΪX��������X��_____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ��ʾ����֪A��B��C��D��������֮���������ת����ϵ������C�ǵ��ʣ�D����Է���������С��������Իش�
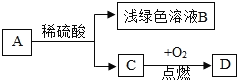
��1��д��A��C��ѧʽ��A��______��C��______��
��2��dz��ɫ��ҺB�����ʵĻ�ѧʽ______��д��A��C�Ļ�ѧ����ʽ______��
��3��д��C��D�Ļ�ѧ����ʽ______��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com