
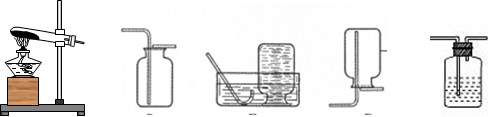
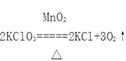 �����ܿ�������ð�����ɿ���һ���ڵ��ܿ��γ�һ��ˮ����
�����ܿ�������ð�����ɿ���һ���ڵ��ܿ��γ�һ��ˮ����

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
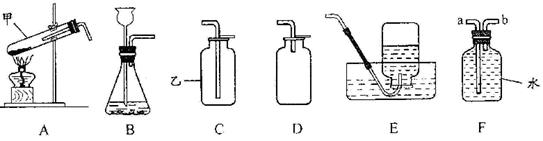
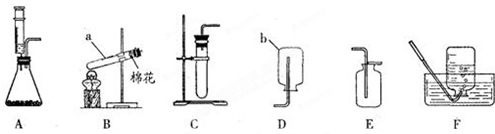
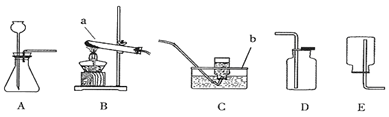
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�������a��ͨ�룬�ռ���O2 |
| B��ƿ��װ�г���ʯ��ˮ������O2���Ƿ�CO2 |
| C��ƿ��װ��NaOH��Һ������CO�л��е�CO2 |
| D����b�˽���Ͳ��ƿ��װ��ˮ�������������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
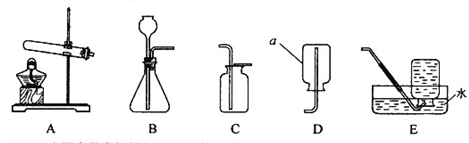
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
 CaCl2 + 2NH3��+ 2H2O
CaCl2 + 2NH3��+ 2H2O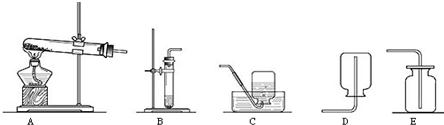
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����

| ���� | þ | ��̼0.05������ | ��̼0.2������ | ��̼0.6������ |
| ȼ��ʱ ������ | ����ȼ�գ����� ҫ�۰⣬���� | ����ȼ�� ���ٻ��� | ����ȼ�� �������� | (δ��) |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����к����ȴ��ˮ�������������� |
| B����к����ȴ��ˮ���������Ķ�����̼ |
| C��ˮ���ܽ����������й������ݳ������� |
| D��ˮ��ϸ������еĹ����б�ɱ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com