
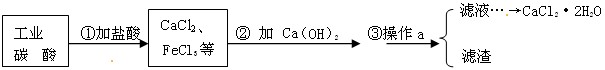
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ҽ���Ȼ��Ƴ����ںϳ�ҩ��Թ�ҵ̼���(������Fe3+������)Ϊԭ��������ˮ���Ȼ���
(CaCl2��2H2O)������ͼ������ʾ��

(1)д���ڢٲ���̼��������ᷴӦ�Ļ�ѧ����ʽ��_____________________��
(2)�ڢڲ����Ƿ�����ѧ�仯��________________(��ǡ���)��
(3)����a��������_________��ʵ���ҽ��иò���ʱ��������������_________
___________________________��
(4)���������������������Σ���Ҫ�����㹻�ĸƣ�д��һ�ֺ����IJ��Ʒ�����_________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ҽ���Ȼ��Ƴ����ںϳ�ҩ��Թ�ҵ̼��ƣ�������Fe3+�����ʣ�Ϊԭ��������ˮ���Ȼ��ƣ�CaCl2·2H2O������������ͼ��ʾ��
|
|
�ټ����� �� ��Ca��OH��2 �۲���a
![]()
![]()
![]() ����
����
��1��д���ڢٲ���̼��������ᷴӦ�Ļ�ѧ����ʽ��————————————————
��2���ڢڲ����Ƿ�����ѧ�仯��——————����ǡ�����
��3������a��������——————————��ʵ���ҽ��иò���ʱ��������������————————————��
��4�����������������������Σ���Ҫ�����㹻�ĸƣ�д��һ�������IJ��Ʒ�����————————————————————————————————————————————————————————————————��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ҽ���Ȼ��Ƴ����ںϳ�ҩ��Թ�ҵ̼��ƣ�������Fe3+�����ʣ�Ϊԭ��������ˮ���Ȼ��ƣ�CaCl2·2H2O������������ͼ��ʾ��
|
|
�ټ����� �� ��Ca��OH��2 �۲���a
![]()
![]()
![]() ����
����
��1��д���ڢٲ���̼��������ᷴӦ�Ļ�ѧ����ʽ��
��2���ڢڲ����Ƿ�����ѧ�仯�� ����ǡ�����
��3������a�������� ��ʵ���ҽ��иò���ʱ�������������� ��
��4�����������������������Σ���Ҫ�����㹻�ĸƣ�д��һ�������IJ��Ʒ����� ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com