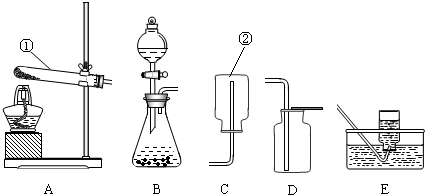
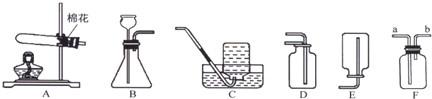
ʵ������ȡ����ij���װ����ͼ��ʾ��������ѧ��֪ʶ�ش���������

��1��д��װ���б�����������ƣ���
�Թ�
�Թ�
����
����©��
����©��
��
��2��ʵ�����ø��������ȡ��������ѡ�õķ���װ����
B
B
������ĸ����д���÷�Ӧ�Ļ�ѧ����ʽ��
��
��3��д��ʵ�����ô���ʯ��ϡ������ȡ������̼�Ļ�ѧ����ʽ��
CaCO3+2HCl�TCaCl2+H2O+CO2��
CaCO3+2HCl�TCaCl2+H2O+CO2��
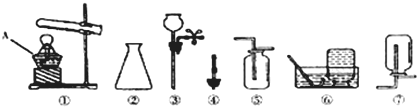
���ݴ�ѡ����ͼ��
CDE
CDE
������ĸ����װһ����ȡ���������̼��װ�ã�
��4��С����ʵ������ȡ������̼��ķ�Һ���ã�ȡ�ϲ���Һ50g����������μ�����������Ϊ26.5%��̼������Һ��������ʵ���õ����ݻ��ͼ������������m��ʵ��õ��ij�����������������������ʾ���Ǽ���̼������Һ���������Լ��㣺
��50g��Һ�к��Ȼ��Ƶ�������
��b���ʾ����Һ���Ȼ��Ƶ�����������




 �ľ�ͼ���ʱ�ȷ�ϵ�д�
�ľ�ͼ���ʱ�ȷ�ϵ�д�