�����������У���ᷢ�֡���ѧ�������ߡ���
��1�������и����ʵ����������֮���Ӧ�Ŀո��У�
a���黯 b���ܶ� c����ζ d����ɫ e���ܽ���
����ϴ�ྫϴ�������۵���꣬����Ϊϴ�ྫ����
a
a
���ã�
�����ں�����
b
b
С�һ�ѧ���ʲ����ã���˳��������������
�����ڹ����Ȼ��ƺ�̼��Ƶ�
e
e
��ͬ�����Կ�����ˮ�������֣�
�����ǿ��Ը��ݾƾ����״�ˮ��
c
c
���Բ�ͬ���������ǣ�
��2��������;��ѡ����Ӧ�������������ǵ������ĸ������Ӧλ�ã�
A���Թ� B���ձ� C������ǯ D������̨ E����ͷ�ι�
������þ��ȼ��ʵ��ʱ�����ڼг�þ����������
C
C
��
���ܽ�϶����Ĺ����õ���������
B
B
��
�ۿ���ֱ���ھƾ��ƻ����ϼ��ȵIJ���������
A
A
��
��ȡ������Һ��ҩƷ��������
E
E
��
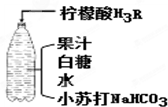
��3������ͼ�䷽������һƿ�����������ˮ�� ����ͼ����Ϣ�ش�
��С�մ��к��еĽ���Ԫ�ص�������
��
��
��
����ˮ�к����������ʵĻ�ѧʽ��
H2O
H2O
��
�۴�ƿ�Dz������壬˵�������ܽ����
ѹǿ
ѹǿ
�йأ�
��������ˮʱ������Ӧ�Ļ�ѧ����ʽΪ��3NaHCO
3+H
3R�TNa
3R+3H
2O+3X������X�Ļ�ѧʽ��
CO2
CO2
��
�ݰ��ǵ���Ҫ�ɷ���C
12H
22O
11������̼��������Ԫ�ص���������
72��11
72��11
��

 5MgO+X+4CO2������X�Ļ�ѧʽ��______��������Щ��Ϣ�������ƶϳ���þ�ۡ�����һ����;��______��
5MgO+X+4CO2������X�Ļ�ѧʽ��______��������Щ��Ϣ�������ƶϳ���þ�ۡ�����һ����;��______�� 5MgO+X+4CO2������֪��ÿ��X�к���2����ԭ�Ӻ�1����ԭ�ӣ���ˮ����X�Ļ�ѧʽ��H2O����Ϊ��ʽ̼��þ����ȼ��300�漴�ֽ������ɶ�����̼�����Կ���������ȼ�����������ȣ���
5MgO+X+4CO2������֪��ÿ��X�к���2����ԭ�Ӻ�1����ԭ�ӣ���ˮ����X�Ļ�ѧʽ��H2O����Ϊ��ʽ̼��þ����ȼ��300�漴�ֽ������ɶ�����̼�����Կ���������ȼ�����������ȣ���



 �����������У���ᷢ�֡���ѧ�������ߡ���
�����������У���ᷢ�֡���ѧ�������ߡ���