
ʵ������ȡ���������װ������ͼ��ʾ��
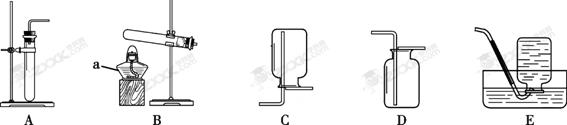
��ش��������⡣
��1��д��������������ƣ�����������������������
��2���ù���������Һ�Ͷ���������ȡ�����Ļ�ѧ����ʽΪ ���ø��������ȡ����ʱ����ѡ�õķ���װ��Ϊ________������ĸ����
��3���ô���ʯ��ϡ������ȡ������̼�Ļ�ѧ����ʽΪ ����װ��________������ĸ���ռ�һƿ������̼����ȼ�ŵ�ľ���ӽ�ƿ�ڣ����۲쵽 ��˵��ƿ���ѳ���������̼��
��4��������ԭ��������һ����̼����������Ӧ����ʵ�����������ͼ��ʾװ�ý���ʵ�飬��ʵ�黹�ɵó��й�һ����̼����ػ�ѧ�����ǣ��û�ѧ����ʽ��ʾ�� ��
 |

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com