
�Ȼ������������Ʒ��Ҳ����Ҫ�Ļ���ԭ�ϡ��ᴿ��������ɳ�Ĵ��Σ�һ�㾭�����²������̣�

��1���������б����õ���һ�������� ������ţ���
A.�в� B.��Ͳ C.�ձ� D.�Թ�
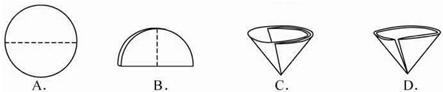
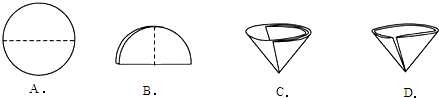
��2������������Ҫ��Բ����ֽ�۵�����������ͼʾ�в��ó��ֵ������� ������ţ���

��3���������г��õ����żܡ��ƾ��ơ�������������ǯ�⣬����Ҫ�õ� ���������ò������������ʳ�ι���ɽ���Ϊ�������ٷɽ���������������ɲ�ȡ
�ȴ�ʩ��
��4��������˺�õ�����Һ��Ȼ���ǣ���д�������Һ���ǵĿ���ԭ��
��дһ������
��5��ʵ������������õľ��Σ������㾫�ε��Ƶ��ʣ������Ƶ��ʽϵͣ������ԭ����
������ţ���
A.ʳ��û��ȫ���ܽ⼴���� B.����ʱʳ�ηɽ�����
C.���������þ��κܳ�ʪ D.������մ�еľ���ûȫ��ת�Ƶ�����ֽ��
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com