
���� Χ�ƻ�ѧʽ�Ͷ�ӦԪ�ص�����������չ��������������㣬�����е�һҪ��������Ԫ�ص����࣬�����Ҫȷ���������ԭ��������
��� �⣺��1��8.8g������̼�к�̼Ԫ�ص�����8.8g��$\frac{12}{12+16��2}$��100%=2.4g��
��2��2.2g������̼��1.4gһ����̼��������к���̼Ԫ�ص�����2.2g��$\frac{12}{12+16��2}$��100%+1.4g��$\frac{12}{12+16}$��100%=1.2g��
��3��һ����̼�Ͷ�����̼�Ļ�����壬����������64%������������һ����̼����������x���������̼����������Ϊ1-x
������Ԫ����ʽ��1-x����$\frac{16��2}{12+16��2}$��100%+x��$\frac{16}{12+16}$��100%=64%
x��56%
��4�������Ͷ�����̼�Ļ������6.0g������������Ԫ��80%��
���Ӧ��̼Ԫ�ص�����Ϊ1-80%=20%��
���Ӧ�Ķ�����̼������=6.0g��20%�£�$\frac{12}{12+16��2}$��100%��=4.4g
�������������������6.0g-4.4g=1.6g��
��5��1.8g̼��ȫת��Ϊһ����̼�Ͷ�����̼��������������̼������Ϊ4.4g��
�������̼��̼Ԫ�ص�����Ϊ4.4g��$\frac{12}{12+16��2}$��100%=1.2g
��ת��Ϊһ����̼��̼Ԫ�ص�����Ϊ1.8g-1.2g=0.6g
��ͬʱ����һ����̼������Ϊ0.6g�£�$\frac{12}{12+16}$��100%��=1.4g��
�𣺣�1��8.8g������̼�к�̼Ԫ�ص�����2.4g��
��2��2.2g������̼��1.4gһ����̼��������к���̼Ԫ�ص�����1.2g��
��3��һ����̼�Ͷ�����̼�Ļ�����壬����������64%������������һ����̼�ĺ���56%��
��4�������Ͷ�����̼�Ļ������6.0g������������Ԫ��80%����������������������1.6g��
��5��1.8g̼��ȫת��Ϊһ����̼�Ͷ�����̼��������������̼������Ϊ4.4g����ͬʱ����һ����̼1.4g��
���� ���ݻ����Ԫ������ʱһ��Ҫע�����Ӧ�ɷ��Ƿ��ж�ӦԪ����ɸ��ţ�������͵������У�������һ����̼�Ͷ�����̼��̼������������������̼��


 ����ͬ�����Ծ�ϵ�д�
����ͬ�����Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
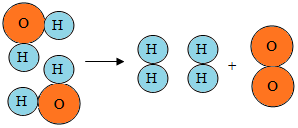 ����ͼˮ���ӷֽ�ʾ��ͼ�л��������Ϣ�����в���ȷ���ǣ�������
����ͼˮ���ӷֽ�ʾ��ͼ�л��������Ϣ�����в���ȷ���ǣ�������| A�� | �ڻ�ѧ�仯�У����ӿɷ�ԭ�Ӳ��ɷ� | |
| B�� | ��ѧ��Ӧǰ��Ԫ�ص������ | |
| C�� | ˮ����������������ɵ� | |
| D�� | 1��ˮ������2����ԭ�Ӻ�1����ԭ�ӹ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
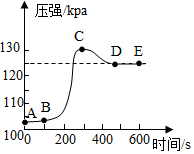 С����δ��ɰ����ĥ����������ʢ��ϡ������ܱ������У���ѹǿ�������������������ѹǿ�ͷ�Ӧʱ��ı仯������ͼ��ʾ�����з����в���ȷ���ǣ�������
С����δ��ɰ����ĥ����������ʢ��ϡ������ܱ������У���ѹǿ�������������������ѹǿ�ͷ�Ӧʱ��ı仯������ͼ��ʾ�����з����в���ȷ���ǣ�������| A�� | AB�εĻ�ѧ��Ӧ�ǣ�Al2O3+6HCl�T2AlCl3+3H2O | |
| B�� | C�����ɵ�������������D�� | |
| C�� | CD��ѹǿ�仯����ҪӰ�������������¶� | |
| D�� | D��E���㴦������ѹǿ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
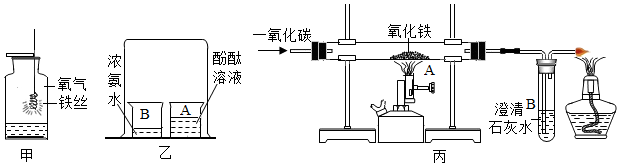
 ��ͼΪ�ס������ֹ������ʵ��ܽ�����ߣ���ش��������⣺
��ͼΪ�ס������ֹ������ʵ��ܽ�����ߣ���ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
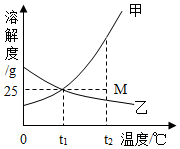
| A�� | ������̼������������Һ | B�� | �������ƺ�ϡ���� | ||
| C�� | ̼���ƹ����ϡ���� | D�� | ��������ͭ��Һ |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com