
��6�֣�ijͬѧΪ���о�������ԭ�����ⶨ����������������һ����̼����������Ӧ������ͼ����ʵ�顣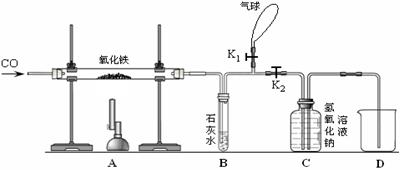
��1�����ȣ���ͬѧ������������������Ȼ�����ʵ�飬���ȴ�K1���ر�K2��ͨ��һ����̼����Ŀ�����ž��������ڵĿ�������ֹ����ʱ��һ����̼����������ը��Ȼ��ر�K1����K2�����ȣ���Ӧһ��ʱ�����ȴ���ٴγ�����������������ֹ�������������12�ˣ���������������Ϊ_____���������з�����Ӧ�Ļ�ѧ����ʽΪ_________________��
��2��װ��B�з�Ӧ�Ļ�ѧ����ʽ��________________________________��
��3��װ��C������������________________________________________________��
��4��ʵ��������ձ��ڵ���Һ�к��е�������_________________��д��ѧʽ����
��1��28�� 3CO + Fe2O3 ���� 2Fe+3CO2
��2��CO2 + Ca(OH)2="==" Ca2CO3��+ H2O
��3�����շ�Ӧ���ɵĶ�����̼��
ͬʱ��ֹһ����̼�ݳ���Ⱦ���������ռ��ϴ�����CO��
��4��NaOH��Na2CO3
���������������1������һ����̼�ڸ���������������Ӧ�������Ͷ�����̼�ķ�Ӧ��֪����Ӧ������������ٵ�������Ϊ����������Ԫ�ص�������������������������Ԫ�ص������ȣ�����Ԫ�ص������������������������
������Fe2O3��Fe��OԪ�ص�������=��56��2������16��3��=7��3�������������֪������������Ԫ������Ϊ12gʱ������Ԫ�ص�����=12g��7��3=28g���ڸ��������£�һ����̼����������Ӧ�������Ͷ�����̼���ʷ�Ӧ�Ļ�ѧ����ʽΪ3CO+Fe2O3 ���� 2Fe+3CO2��
��2��װ��B�ڵij���ʯ��ˮ���뷴Ӧ���ɵĶ�����̼��Ӧ�����ɲ�����ˮ��̼��Ƴ�����ˮ���ʷ�Ӧ�Ļ�ѧ����ʽΪCO2+Ca(OH)2=Ca2CO3��+H2O��
��3�������������Ƶ����ʷ�����װ��C�е�����������Һ����β���еĶ�����̼ȫ����ȥ��ͬʱ���ܰ�ʣ���һ����̼�����ռ����Է�ֹһ����̼�ų�����Ⱦ������
��4�����������������ն�����̼�������̼���ƣ��ʿ��жϴ�װ��C���ų�����Һ����δ��Ӧ���������ƣ����������̼��Ӧ���ɵ�̼���ơ�
���㣺һ����̼��ԭ����������Ļ�ѧ���ʣ�Ԫ�������ȵļ��㣬��д��ѧ����ʽ
���������ö�һ����̼��ԭ��������Ӧ���������⣬�ƶϸĽ�װ���и�װ�õ����ã��ǽ�����������һ�㷽����


 ��˼ά������ҵ���ټ��ִ�ѧ������ϵ�д�
��˼ά������ҵ���ټ��ִ�ѧ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�챱�����������п�һģ��ѧ�Ծ��������棩 ���ͣ�̽����
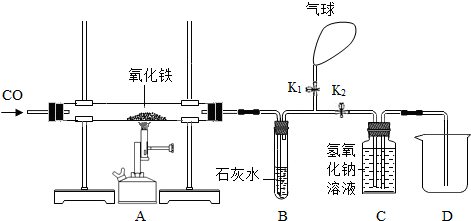
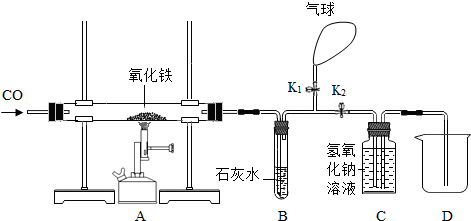
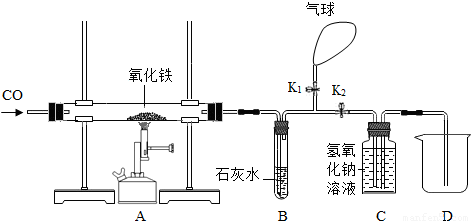
��6�֣�ijͬѧΪ���о�������ԭ�����ⶨ����������������һ����̼����������Ӧ������ͼ����ʵ�顣

��1�����ȣ���ͬѧ������������������Ȼ�����ʵ�飬���ȴ�K1���ر�K2��ͨ��һ����̼����Ŀ�����ž��������ڵĿ�������ֹ����ʱ��һ����̼����������ը��Ȼ��ر�K1����K2�����ȣ���Ӧһ��ʱ�����ȴ���ٴγ�����������������ֹ�������������12�ˣ���������������Ϊ_____���������з�����Ӧ�Ļ�ѧ����ʽΪ_________________��
��2��װ��B�з�Ӧ�Ļ�ѧ����ʽ��________________________________��
��3��װ��C������������________________________________________________��
��4��ʵ��������ձ��ڵ���Һ�к��е�������_________________��д��ѧʽ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�걱�����������п���ѧһģ�Ծ��������棩 ���ͣ������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com