
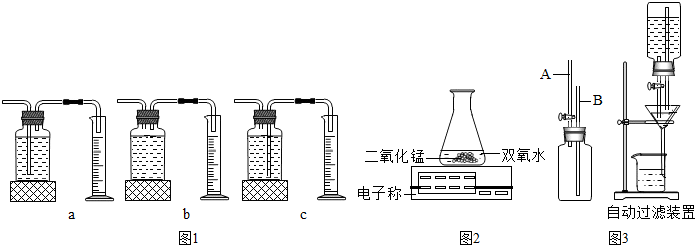
ijͬѧ������ø��������ȡ���ռ�������װ����ͼ��
��1��д��ͼ�б�����ŵ��������ƣ�
��
�ƾ���
�ƾ���
��
����ƿ
����ƿ
��
����̨
����̨
��
��2��ָ������ȡװ�ã���ߣ��е��������Դ���
��
�Թܿ�û����������б
�Թܿ�û����������б
����
�Թܿ�û��һ����
�Թܿ�û��һ����
��
�Ӿƾ�����δ��ȼ�Ƕȿ������ռ�װ���л���������������
��
����ƿ��ˮû����
����ƿ��ˮû����
����
û��ʼ���ȾͰѵ����쵽����ƿ��
û��ʼ���ȾͰѵ����쵽����ƿ��
��
��3����ȡ�����IJ��������У�A�����Թ���װ�������� B�������Թ� C�����װ�������� D�������е��ܵ���Ƥ�������Թܣ����̶�������̨�� E������������ʢ��ˮ�ļ���ƿ�� F���Թܿڷ�һ��������ȷ����˳����
CAFDBE
CAFDBE
��
��4����Ӧ�����ֱ���ʽ��
�������
�����+��������+����
�������
�����+��������+����
��
��5��ʵ����Ϻ�Ӧ��
�ѵ����Ƴ�ˮ��
�ѵ����Ƴ�ˮ��
����
Ϩ��ƾ���
Ϩ��ƾ���
���Է�ֹ
ˮ�������Թܣ�ʹ�Թ�ը��
ˮ�������Թܣ�ʹ�Թ�ը��
��

 ijͬѧ������ø��������ȡ���ռ�������װ����ͼ��
ijͬѧ������ø��������ȡ���ռ�������װ����ͼ��


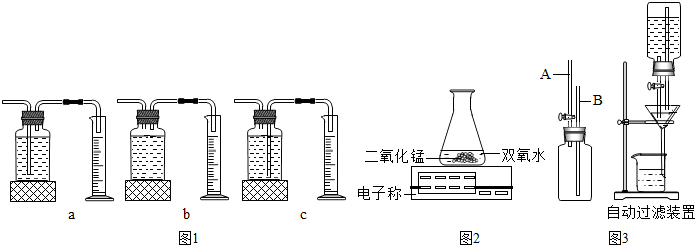
 2KCl+3O2����CuOҲ��������طֽ�Ĵ�������ͬѧΪ̽�����������������طֽ����ʵ�Ӱ�죬��������¶Ա����飺
2KCl+3O2����CuOҲ��������طֽ�Ĵ�������ͬѧΪ̽�����������������طֽ����ʵ�Ӱ�죬��������¶Ա����飺