
��ѧ�����������ߣ��������ǵ�����ϢϢ��أ�
��1����ӡ��������ɱ�������֡����͡���ѡ���ʵ���������գ�
�ٿ���������ҽ����е������ �����ڿ������˹���������� ����
�ۿ����ڳ�ȥ�·������۵����� �����ܿ����������������� ����
��2������H��C��O��Ca���ֳ�����Ԫ�أ���ѡ�����е�Ԫ��д����������Ҫ������ʸ�һ�֣��û�ѧʽ��ʾ����
�ٳ��õ��ܼ��� �����ڴ���ʯ����Ҫ�ɷ��� ����
����Ȼ������Ҫ�ɷ��� ��������ˮ�к��е�һ������ ����
��1������֣��ɱ������ͣ���������2����H2O����CaCO3����CH4����H2CO3��
���������������1��������ѧ���ʲ����ã���������������������ֲ������⣬�����Ƴɲ;�ҽ����е���������ߣ��ɱ��Ƕ�����̼�Ĺ�̬�����������ȣ��������˹����꣬�����Ƕ����л���Ļ������ܽ�������ۣ���2������˵���ж����ʣ�ѡ��Ԫ����д���ɣ��ٳ��õ��ܼ���ˮ����ѧʽΪH2O���ڴ���ʯ����Ҫ�ɷ���̼��ƣ���ѧ��ΪCaCO3������Ȼ������Ҫ�ɷ��Ǽ��飬��ѧʽΪCH4��������ˮ�к��е�һ������̼�� H2CO3��
���㣺�����֣������������;��������̼����;���黯�������黯���ã���ѧʽ����д�����壮


 �Ƹ�С״Ԫ�������������ϵ�д�
�Ƹ�С״Ԫ�������������ϵ�д� ����һ������ܼƻ�ϵ�д�
����һ������ܼƻ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��1��N(NO2)2�ǿ�ѧ��2011�귢�ֵ�һ�����ͻ��ȼ�ϡ�N(NO2)2����Է��������� ��N(NO2)2�и�Ԫ�ص��������� �� N(NO2)2����Ԫ�ص����������� (����һλС��)�� N(NO2)2���ɷ��ӹ��ɵ����ʣ�1��N(NO2)2�����к��е�ԭ������ ��N(NO2)2�����и�ԭ�ӵĸ������ǣ� ��
��2��������������ȼ�յĻ�ѧ����ʽ�ǣ�H2+ Cl2 2HCl �����ʽ�Ӳ��������˻�ѧ��Ӧ�ķ�Ӧ���� ���������� ����Ӧ������ ������ʾ�˲��뷴Ӧ�ĸ�����֮���������ϵ��ÿ �������������� �������������ڵ�ȼ��������ǡ����ȫ��Ӧ���� ���������Ȼ������塣
2HCl �����ʽ�Ӳ��������˻�ѧ��Ӧ�ķ�Ӧ���� ���������� ����Ӧ������ ������ʾ�˲��뷴Ӧ�ĸ�����֮���������ϵ��ÿ �������������� �������������ڵ�ȼ��������ǡ����ȫ��Ӧ���� ���������Ȼ������塣
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����ѧ���������ʣ�������������Ũ���ᡢ��ʯ�ҡ���ʯ�ҡ�����صȣ������������ѡ���ʵ���������գ�Ҫ��д����ѧʽ����
��1������ȫ�����̽����������� ��
��2������������������Ĺ����ǣ��� ����
��3�������������Ϸʵ��ǣ��� ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��һ����̼��������̼�������������Ͷ���������ѡ���ʵ��������û�ѧʽ��գ�
��1��������̬�����Ҿ��п�ȼ�Ե������� ��
��2���ɳ�����Ƶ������� ��
��3������������������� ��
��4����������Ѫ�쵰��ϵ��ж������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�˽����ʵ���;���������Ǹ��õ�����밴����Ҫ��ѡ����ʵ����ʣ���������գ�
������ ��ϡ���� ����ʯ�� ��̼�����
��1����֧��ȼ�յ����� ����
��2������������������������ ����
��3����ũҵ�������ʵ����� ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
д�����з�Ӧ�����ֱ���ʽ
a������������ȼ�գ�
b�����ڿ�����ȼ�գ�
c�����ȸ��������ȡ������
d���ù���������ȡ������
�������ڻ��Ϸ�Ӧ���� �����ڷֽⷴӦ���� ������������Ӧ���� ������ţ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�����������ʣ�����̿����ۡ����ۡ����������������������顢һ����̼��������̼���ƾ��������ǣ��밴����Ҫ���仯ѧʽ����ո��ڣ�ÿ����һ�����ʣ���
��1������������ѧ���ʵ���С������ ����
��2��ú������Ҫ�ɷ��� ����
��3��ʳ�����������Ϊ������֯�ṩӪ������������ ����
��4������������ЧӦ������Ҫԭ��֮һ�Ǵ�����̼���ʵ�ȼ�գ����´������� ���ĺ�������Ϊ�˼����������������ȼ������ ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����ѧ֪ʶϵͳ���������ڶ��������ʶ��������������йط�����������ۡ�
��1����������ɸ�������ɺ����ʽ��з��࣮��K��H��O��N����Ԫ��������ѡ��������ɺ��ʵ����ʣ����仯ѧʽ�ֱ�����ͼ1�Тڡ��ĺ����ϡ�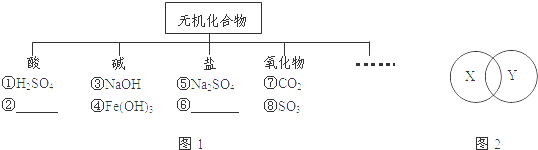
�뽫����ۡ������ּ�������࣬�ɷ�Ϊ�� ����
��2����ѧ��Ӧ֮�䡢��ѧ����֮����а��������С�����ȹ�ϵ���±���X��Y����ͼ2��ʾ��ϵ��
���� ����ѡ�����б���ѡ���
| | A | B | C | D |
| X | ���Ϸ�Ӧ | �û���Ӧ | ������ | ���� |
| Y | ������Ӧ | ���ֽⷴӦ | ������ | ̼���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��1�����ʵ��ķ��ź������Լ���ѧʽ���
�ٱ���ˮ�Ļ�ѧ���ʵ���С���� ������ͭ�е�������
�۹�����������Ԫ�صĻ��ϼ� ������� �������
��2��д�����з�Ӧ�Ļ�ѧ����ʽ
��ʵ�����ù���������ȡ����
��ľ̿���»�ԭ����ͭ
���д̼�����ζ�������ɵĻ��Ϸ�Ӧ
����ˮ���ĵĻ��Ϸ�Ӧ
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com