
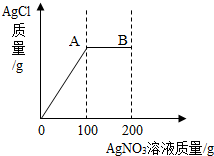
��2.34gNaCl��������103.4gˮ�еõ���������Һ������������Һ��С�ĵ���200g AgNO3��Һ��ʵ������У����ɵ�AgCl������������AgNO3��Һ��������ϵ������ͼ��ʾ����ʾ��NaCl + AgNO3 = AgCl��+ NaNO3 ����

��1������A�㴦����NaNO3��������
��2������B����Һ��AgNO3���������������������������������������С�����һλ)
��1���⣺��A�㴦����NaNO3������Ϊx
AgNO3 + NaCl = AgCl�� + NaNO3
58.5�� 85
2.34g ��x
x = 3.4g
��2����A�㴦��ӦAgNO3������Ϊy������AgCl������Ϊz
AgNO3 + NaCl = AgCl�� + NaNO3
170������ 58.5 143.5
y ��������2.34g ��z
y = 6.8g
z = 5.74g
m��B����Һ��=2.34g +103.4g + 200g - 5.74g = 300g
B����ҺAgNO3�������������� = �� 100% = 2.3%
���������������ո��֣�
��A�㴦����NaNO3������Ϊ3.4g��B����ҺAgNO3��������������Ϊ2.3%��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��2.34gNaCl��������103.4gˮ�еõ���������Һ������������Һ��С�ĵ���200g AgNO3��Һ��ʵ������У����ɵ�AgCl������������AgNO3��Һ��������ϵ��ͼ��ʾ����ʾ��NaCl+AgNO3=AgCl��+NaNO3 ����
��2.34gNaCl��������103.4gˮ�еõ���������Һ������������Һ��С�ĵ���200g AgNO3��Һ��ʵ������У����ɵ�AgCl������������AgNO3��Һ��������ϵ��ͼ��ʾ����ʾ��NaCl+AgNO3=AgCl��+NaNO3 �����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011����б�ҵ��ѧ���ԣ��������־�����ѧ ���ͣ�������
��7�֣���2.34gNaCl��������103.4gˮ�еõ���������Һ������������Һ��С�ĵ���200g AgNO3��Һ��ʵ������У����ɵ�AgCl������������AgNO3 ��Һ��������ϵ����ͼ��ʾ����ʾ��NaCl + AgNO3 =" AgCl��+" NaNO3����
��Һ��������ϵ����ͼ��ʾ����ʾ��NaCl + AgNO3 =" AgCl��+" NaNO3����
��1������A�㴦����NaNO3��������
��2������B����Һ��AgNO3����������������
�����������������������С�����һλ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�������������п����� ���ͣ�������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ��㶫ʡտ��һ�о��꼶���£���7���¿���ѧ�Ծ��������棩 ���ͣ������

�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com