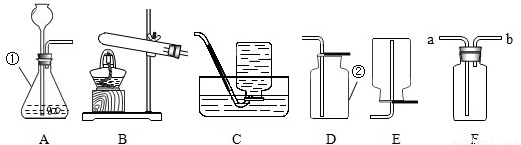
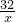ʵ������ȡ����ʱ�����õ�����ͼ��ʾ��װ�ã�ij��ȤС���ͬѧҪ��������װ�ý�������̽��ѧϰ�������һͬ�μӲ��ش��������⣮
��1��д��������������ƣ���
��ƿ
��ƿ
�� ��
����ƿ
����ƿ
��
��2������Ҫ��Aװ����ȡ��������д���÷�Ӧ�Ļ�ѧ����ʽ
��
��3��ΰΰҪ�ø��������ȡ�ϴ�������������Ӧ�Ļ�ѧ����ʽΪ
����Ӧѡ�����ȡװ����
BC
BC
����װ�ô��ڲ���֮�������ܻᵼ��
�������С�������뵼����
�������С�������뵼����
��
��4��ǿǿ��Ϊ��Fװ�ü����ռ����廹�ܸ������壮��Ҫ����ˮ�����ռ����������ڹ��ƿ��װ��ˮ��������Ӧ��
b
b
���a����b������ͨ�룻��Ҫ�������������ڹ��ƿ��ʢװ����
Ũ����
Ũ����
���������a��ͨ��b�˵�����
��5��ͮͮ����ͼѡ�����ʵ�װ�ã��ɹ����Ƶ��˶�����̼����ѡ�õ���ȡװ����
AD
AD
��ɣ���ѷ�Ӧ�Ļ�ѧ����ʽ
CaCO3+2HCl=CaCl2+H2O+CO2��
CaCO3+2HCl=CaCl2+H2O+CO2��
��
��6���Ѽ�Ҫ����ͮͮ�Ƶõ�������Ƕ�����̼������Fװ���м����Լ�
ʯ��ˮ
ʯ��ˮ
����������aͨ�룬��ʱ���ƿ�ڷ�����Ӧ�Ļ�ѧ����ʽΪ
CO2+Ca��OH��2=CaCO3��+H2O
CO2+Ca��OH��2=CaCO3��+H2O
�����Ѽѵ�ʵ�鲢û�в���Ԥ�ڵ�����ͮͮ�ͼѼѶ�ȷ�ϸ��������Լ�û�б��ʻ�ʧЧʱ����������ҳ����´˽���Ŀ��ܵ�ԭ��
�ռ��Ķ�����̼�л����Ȼ�������
�ռ��Ķ�����̼�л����Ȼ�������
��


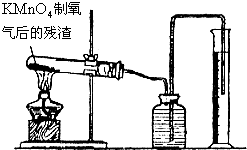
 K2MnO4+MnO2+O2��
K2MnO4+MnO2+O2�� =
=