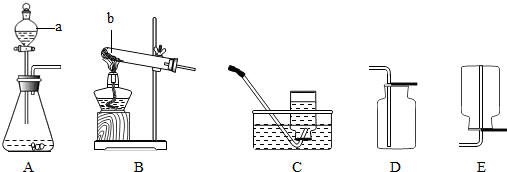
ͨ����ѧѧϰ�����Ѿ�������ʵ������ȡ������йع��ɣ�����������װ��ͼ�ش����⣺��������ʾ����ѡװ�þ�����ţ�

��1��ʵ������ȡO
2 ʱӦѡ�õķ���װ����
A��B
A��B
װ�ã���Ӧ�Ļ�ѧ����ʽΪ
2KClO
32KCl+3O
2����2H
2O
22H
2O+O
2����
2KClO
32KCl+3O
2����2H
2O
22H
2O+O
2����
��
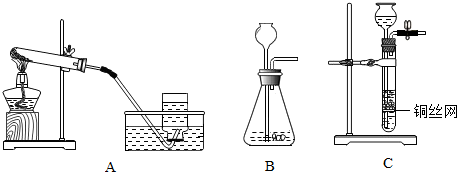
��2��������E�ռ���������Ϊ
�������ܶȱȿ�����
�������ܶȱȿ�����
�����������ķ�����
�������ǵ�ľ�����ڼ���ƿ�ڣ���ľ����ȼ��˵���ռ�����
�������ǵ�ľ�����ڼ���ƿ�ڣ���ľ����ȼ��˵���ռ�����
��
��3��ʵ������ȡCO
2ʱ��ѡ�õķ���װ����ʢ�ŵ�ҩƷ��
ϡ����
ϡ����
��
����ʯ��ʯ��ʯ
����ʯ��ʯ��ʯ
���ӳ���©����ע��Һ���ῴ��ʲô����
����ʯ��ʯ��ʯ��������������
����ʯ��ʯ��ʯ��������������
��Ӧ�Ļ�ѧ����ʽ�ǣ�
CaCO3+2HCl=CaCl2+H2O+CO2��
CaCO3+2HCl=CaCl2+H2O+CO2��
��
��4����μ����ռ����������CO
2����ƿ�ڵ�������ʯ��ˮ���������ʯ��ˮ����ǣ����Ƕ�����̼
����ƿ�ڵ�������ʯ��ˮ���������ʯ��ˮ����ǣ����Ƕ�����̼
��




 ������ÿ�ʱ�Ż���ҵϵ�д�
������ÿ�ʱ�Ż���ҵϵ�д�
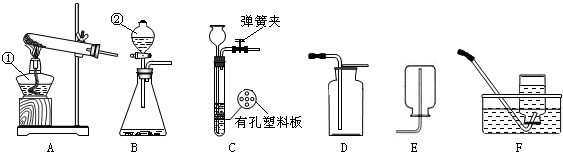
 ͨ����ѧѧϰ�����Ѿ�������ʵ������ȡ�����һЩ���ɣ�������ͼ�ش����⣺
ͨ����ѧѧϰ�����Ѿ�������ʵ������ȡ�����һЩ���ɣ�������ͼ�ش����⣺