��2011?���ݣ��ֻ�ԭ������һ����Ҫ�Ļ���ԭ�ϣ�ij��ȤС�������������о���
[�����Ʊ�]�����̷��Ʊ�����ԭ���۵Ĺ����������£�

�ֻ�ԭ�����л��������������������Fe
3C�����������ڸ����½�һ����ԭ���䷴Ӧ����ʽΪ��Fe
xO
Y+yH
2xFe+yH
2O Fe
3C+2H
23Fe+CH
4��1��д����������������CO��Ӧ�Ļ�ѧ����ʽ
��
��2�������мӽ�̿�����ó��˿�������CO�⣬����
�ṩ����
�ṩ����
��
[�����ⶨ]Ϊ�õ�����ԭ���۲��ⶨ�ֻ�ԭ����������̼Ԫ�ص�����������������װ�ý������飬��֪ 3CH
4+4Fe
2O
33CO
2+6H
2O+8Fe
������ÿ����Ӧ����ȫ�Ҳ�����װ����ԭ���п����Բⶨ�����Ӱ�죩��
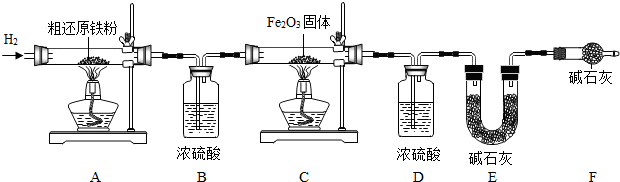
��3����Ҫʵ�鲽�����£�
�ٰ�˳����װ���������װ�õ������ԣ�������Ʒ�ͱ�Ҫװ�õ��������ڵ�ȼA���ƾ��ƣ��ۻ���ͨ�봿�������H
2���ܵ�ȼC���ƾ��ƣ��ݷֱ�Ϩ��A��C���ƾ��ƣ����ٻ���ͨ������H
2�����ٴγ�����Ҫװ�õ�������
�������Ⱥ�˳���Ǣ٢�
�ܢ�
�ܢ�
�ݢޢߣ�����ţ���
��4������۵�Ŀ����
��ȥװ���ڵ���������ֹ������ը
��ȥװ���ڵ���������ֹ������ը
����֤�ò���Ŀ�Ĵﵽ��ʵ�鷽����
�ռ�β������ȼ�ŵľƾ��ƣ�������
�ռ�β������ȼ�ŵľƾ��ƣ�������
�������Ŀ����
��ֹ���ɵĻ�ԭ�����ٴα��������𱣻�����
��ֹ���ɵĻ�ԭ�����ٴα��������𱣻�����
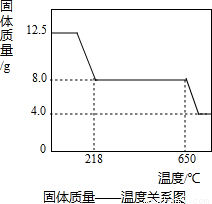
��5����װ��D��E�ֱ�����mg��ng����m��n�Ĺ�ϵΪ
C
C
A.11m=9n B.11m��9n C.11m��9n
��ȱ��װ��D����������Ԫ�ص�����������
����
����
���ƫ����ƫС�����ڡ�����ͬ����̼Ԫ�ص�����������
ƫ��
ƫ��
��
��6���ֻ�ԭ������Ʒ������Ϊ10.000g��װ��B��E�ֱ�����0.180g��0.220g��������Ʒ������̼Ԫ�ص�����������Ҫ�������̣���

 ��2011?���ݣ�ijѧϰС���������������о���
��2011?���ݣ�ijѧϰС���������������о���




 ��2011?���ݣ���һ������ ��ı�ǩ��ͼ��
��2011?���ݣ���һ������ ��ı�ǩ��ͼ��