
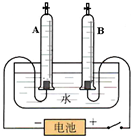
 ijУ����С���ͬѧ������ˮ�ĵ��ʵ��̽��ˮ����ɣ�������ȡ192.7mLˮ��ˮ���ܶ�Ϊ1.00g/cm3��������ˮ�м�����7.3g�������ƹ��壬����ܽ������ͼ��ʾ��װ�ý���ʵ�飬��ͨ��Դ����A���ռ���22.3mL���壨�����ܶ�Ϊ0.09g/L��ʱ��ֹͣʵ�飮��ͨ������ش�
ijУ����С���ͬѧ������ˮ�ĵ��ʵ��̽��ˮ����ɣ�������ȡ192.7mLˮ��ˮ���ܶ�Ϊ1.00g/cm3��������ˮ�м�����7.3g�������ƹ��壬����ܽ������ͼ��ʾ��װ�ý���ʵ�飬��ͨ��Դ����A���ռ���22.3mL���壨�����ܶ�Ϊ0.09g/L��ʱ��ֹͣʵ�飮��ͨ������ش�| 7.3g |
| 192.7g+7.3g-0.018g |
| ||
| 36 |
| x |
| 4 |
| 0.002g |
| 7.3g |
| 192.7g+7.3g-0.018g |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��2012?���������ж���ɽ��ʢ��ʯ��ʯ��ijУ����С��Ϊ�˽�ʯ��ʯ��Դ��Ʒ�ʣ��������ַ����Ե���ʯ�Ľ��л�ѧ������
��2012?���������ж���ɽ��ʢ��ʯ��ʯ��ijУ����С��Ϊ�˽�ʯ��ʯ��Դ��Ʒ�ʣ��������ַ����Ե���ʯ�Ľ��л�ѧ�������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ���� | ʵ�鷽���Ͳ��� | ʵ������ | ʵ����� | ��ʦ���� |
| �� | ��1����ɫ��Һ�м�BaCl2����2����ɫ�����м�ϡHNO3 | ������ɫ�������������ܽ� | ����SO42- | ������ |
| �� | ��1����ɫ��Һ�м�Ba��NO3��2����2����ɫ�����м�ϡH2SO4 | ������ɫ���������д�����ɫ���� | ����SO42- | ������ |
| ����Ϊ | ���������������ǣ�����Һ���� Ag+ Ag+ ͬ������������ | |||
| ���������������ǣ�����Һ���� CO32- CO32- ͬ������������ | ||||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ʴ���
| ���� | ʵ�鷽���Ͳ��� | ʵ������ | ʵ����� | ��ʦ���� |
| �� | ��1����ɫ��Һ�м�BaCl2����2����ɫ�����м�ϡHNO3 | ������ɫ�������������ܽ� | ����SO42- | ������ |
| �� | ��1����ɫ��Һ�м�Ba��NO3��2����2����ɫ�����м�ϡH2SO4 | ������ɫ���������д�����ɫ���� | ����SO42- | ������ |
| ����Ϊ | ���������������ǣ�����Һ����______ͬ������������ | |||
| ���������������ǣ�����Һ����______ͬ������������ | ||||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���ж���ɽ��ʢ�� ʯ��ʯ��ijУ����С��Ϊ�˽�ʯ��ʯ��Դ��Ʒ�ʣ��������ַ����Ե���ʯ�Ľ��л�ѧ������
ʯ��ʯ��ijУ����С��Ϊ�˽�ʯ��ʯ��Դ��Ʒ�ʣ��������ַ����Ե���ʯ�Ľ��л�ѧ������
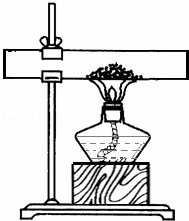
����һ��ȡ10gʯ��ʯ��ĩ��Ʒ������ͼ��ʾװ�ã���ּ����������㶨����ȴ��Ƶù�������Ϊ8.12g��
����������ȡ10gͬ�ʵ�ʯ��ʯ������Ʒ�������ձ��м�����ϡ���ᣬ��ַ�Ӧ��Ƶû�����������������4.27g��
������ϣ�
����ʯ��ʯ���е���Ҫ����Ϊ�������裬�仯ѧ�����ȶ������Ȳ��ֽ��Ҳ������ᷢ ����ѧ��Ӧ��
����ѧ��Ӧ��
��������ַ�����õ�ʯ��ʯ��̼��Ƶ�����������
��1������һ��̼��Ƶ������������� ����
��2����������̼��Ƶ������������� ��������ȷ��0.1%��
�Ƚ������������Ľ�����������������ϴ�Ŀ���ԭ��
�� ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2004�����ʡ�Ƹ��л�����ѧ�п���ѧģ���Ծ��������棩 ���ͣ������
| ���� | ʵ�鷽���Ͳ��� | ʵ������ | ʵ����� | ��ʦ���� |
| �� | ��1����ɫ��Һ�м�BaCl2����2����ɫ�����м�ϡHNO3 | ������ɫ�������������ܽ� | ����SO42- | ������ |
| �� | ��1����ɫ��Һ�м�Ba��NO3��2����2����ɫ�����м�ϡH2SO4 | ������ɫ���������д�����ɫ���� | ����SO42- | ������ |
| ����Ϊ | ���������������ǣ�����Һ���� ͬ������������ | |||
| ���������������ǣ�����Һ���� ͬ������������ | ||||
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com